The endogenous nitric oxide mediates selenium-induced phytotoxicity by promoting ROS generation in Brassica rapa
- PMID: 25333984
- PMCID: PMC4204988
- DOI: 10.1371/journal.pone.0110901
The endogenous nitric oxide mediates selenium-induced phytotoxicity by promoting ROS generation in Brassica rapa
Abstract
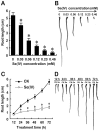
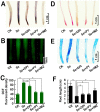
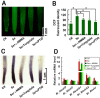
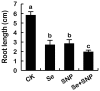
Selenium (Se) is suggested as an emerging pollutant in agricultural environment because of the increasing anthropogenic release of Se, which in turn results in phytotoxicity. The most common consequence of Se-induced toxicity in plants is oxidative injury, but how Se induces reactive oxygen species (ROS) burst remains unclear. In this work, histofluorescent staining was applied to monitor the dynamics of ROS and nitric oxide (NO) in the root of Brassica rapa under Se(IV) stress. Se(IV)-induced faster accumulation of NO than ROS. Both NO and ROS accumulation were positively correlated with Se(IV)-induced inhibition of root growth. The NO accumulation was nitrate reductase (NR)- and nitric oxide synthase (NOS)-dependent while ROS accumulation was NADPH oxidase-dependent. The removal of NO by NR inhibitor, NOS inhibitor, and NO scavenger could alleviate Se(IV)-induced expression of Br_Rbohs coding for NADPH oxidase and the following ROS accumulation in roots, which further resulted in the amelioration of Se(IV)-induced oxidative injury and growth inhibition. Thus, we proposed that the endogenous NO played a toxic role in B. rapa under Se(IV) stress by triggering ROS burst. Such findings can be used to evaluate the toxic effects of Se contamination on crop plants.
Conflict of interest statement
Figures





References
-
- Zhu YG, Pilon-Smits EA, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14: 436–442. - PubMed
-
- Silva M, Arruda M (2012) Identification of selenium in the leaf protein of sunflowers by a combination of 2D-PAGE and laser ablation ICP-MS. Microchimica Acta 176: 131–136.
-
- Fang Y, Catron B, Zhang Y, Zhao L, Caruso JA, et al. (2010) Distribution and in vitro availability of selenium in selenium-containing storage protein from selenium-enriched rice utilizing optimized extraction. J Agric Food Chem 58: 9731–9738. - PubMed
-
- Hasanuzzaman M, Hossain MA, Fujita M (2011) Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res 143: 1704–1721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

