Near-infrared imaging of adoptive immune cell therapy in breast cancer model using cell membrane labeling
- PMID: 25334026
- PMCID: PMC4204826
- DOI: 10.1371/journal.pone.0109162
Near-infrared imaging of adoptive immune cell therapy in breast cancer model using cell membrane labeling
Abstract
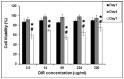
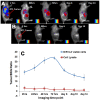
The overall objective of this study is to non-invasively image and assess tumor targeting and retention of directly labeled T-lymphocytes following their adoptive transfer in mice. T-lymphocytes obtained from draining lymph nodes of 4T1 (murine breast cancer cell) sensitized BALB/C mice were activated in-vitro with Bryostatin/Ionomycin for 18 hours, and were grown in the presence of Interleukin-2 for 6 days. T-lymphocytes were then directly labeled with 1,1-dioctadecyltetramethyl indotricarbocyanine Iodide (DiR), a lipophilic near infrared fluorescent dye that labels the cell membrane. Assays for viability, proliferation, and function of labeled T-lymphocytes showed that they were unaffected by DiR labeling. The DiR labeled cells were injected via tail vein in mice bearing 4T1 tumors in the flank. In some cases labeled 4T1 specific T-lymphocytes were injected a week before 4T1 tumor cell implantation. Multi-spectral in vivo fluorescence imaging was done to subtract the autofluorescence and isolate the near infrared signal carried by the T-lymphocytes. In recipient mice with established 4T1 tumors, labeled 4T1 specific T-lymphocytes showed marked tumor retention, which peaked 6 days post infusion and persisted at the tumor site for up to 3 weeks. When 4T1 tumor cells were implanted 1-week post-infusion of labeled T-lymphocytes, T-lymphocytes responded to the immunologic challenge and accumulated at the site of 4T1 cell implantation within two hours and the signal persisted for 2 more weeks. Tumor accumulation of labeled 4T1 specific T-lymphocytes was absent in mice bearing Meth A sarcoma tumors. When lysate of 4T1 specific labeled T-lymphocytes was injected into 4T1 tumor bearing mice the near infrared signal was not detected at the tumor site. In conclusion, our validated results confirm that the near infrared signal detected at the tumor site represents the DiR labeled 4T1 specific viable T-lymphocytes and their response to immunologic challenge can be imaged in vivo.
Conflict of interest statement
Figures





References
-
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, et al. (2002) Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A 99: 16168–16173. - PMC - PubMed
-
- Le HK, Graham L, Miller CH, Kmieciak M, Manjili MH, et al. (2009) Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1+ ionomycin in IL-7+IL-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in IL-2. Cancer Immunol Immunother 58: 1565–1576. - PMC - PubMed
-
- Tuttle TM, Bethke KP, Inge TH, Mccrady CW, Pettit GR, et al. (1992) Bryostatin 1-activated T cells can traffic and mediate tumor regression. J Surg Res 52: 543–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

