Why are medical and health-related studies not being published? A systematic review of reasons given by investigators
- PMID: 25335091
- PMCID: PMC4198242
- DOI: 10.1371/journal.pone.0110418
Why are medical and health-related studies not being published? A systematic review of reasons given by investigators
Abstract
Objective: About half of medical and health-related studies are not published. We conducted a systematic review of reports on reasons given by investigators for not publishing their studies in peer-reviewed journals.
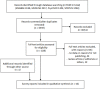
Methods: MEDLINE, EMBASE, PsycINFO, and SCOPUS (until 13/09/2013), and references of identified articles were searched to identify reports of surveys that provided data on reasons given by investigators for not publishing studies. The proportion of non-submission and reasons for non-publication was calculated using the number of unpublished studies as the denominator. Because of heterogeneity across studies, quantitative pooling was not conducted. Exploratory subgroup analyses were conducted.
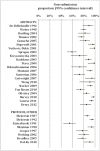
Results: We included 54 survey reports. Data from 38 included reports were available to estimate proportions of at least one reason given for not publishing studies. The proportion of non-submission among unpublished studies ranged from 55% to 100%, with a median of 85%. The reasons given by investigators for not publishing their studies included: lack of time or low priority (median 33%), studies being incomplete (median 15%), study not for publication (median 14%), manuscript in preparation or under review (median 12%), unimportant or negative result (median 12%), poor study quality or design (median 11%), fear of rejection (median 12%), rejection by journals (median 6%), author or co-author problems (median 10%), and sponsor or funder problems (median 9%). In general, the frequency of reasons given for non-publication was not associated with the source of unpublished studies, study design, or time when a survey was conducted.
Conclusions: Non-submission of studies for publication remains the main cause of non-publication of studies. Measures to reduce non-publication of studies and alternative models of research dissemination need to be developed to address the main reasons given by investigators for not publishing their studies, such as lack of time or low priority and fear of being rejected by journals.
Conflict of interest statement
Figures
References
-
- World Medical Association (2013) World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 310: 2191–2194. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources