Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies
- PMID: 25335105
- PMCID: PMC4204810
- DOI: 10.1371/journal.pmed.1001748
Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies
Abstract
Background: The term "atopic march" has been used to imply a natural progression of a cascade of symptoms from eczema to asthma and rhinitis through childhood. We hypothesize that this expression does not adequately describe the natural history of eczema, wheeze, and rhinitis during childhood. We propose that this paradigm arose from cross-sectional analyses of longitudinal studies, and may reflect a population pattern that may not predominate at the individual level.
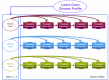
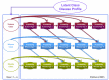
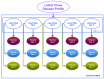
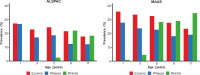
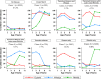
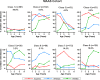
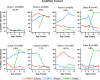
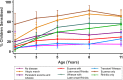
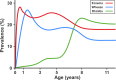
Methods and findings: Data from 9,801 children in two population-based birth cohorts were used to determine individual profiles of eczema, wheeze, and rhinitis and whether the manifestations of these symptoms followed an atopic march pattern. Children were assessed at ages 1, 3, 5, 8, and 11 y. We used Bayesian machine learning methods to identify distinct latent classes based on individual profiles of eczema, wheeze, and rhinitis. This approach allowed us to identify groups of children with similar patterns of eczema, wheeze, and rhinitis over time. Using a latent disease profile model, the data were best described by eight latent classes: no disease (51.3%), atopic march (3.1%), persistent eczema and wheeze (2.7%), persistent eczema with later-onset rhinitis (4.7%), persistent wheeze with later-onset rhinitis (5.7%), transient wheeze (7.7%), eczema only (15.3%), and rhinitis only (9.6%). When latent variable modelling was carried out separately for the two cohorts, similar results were obtained. Highly concordant patterns of sensitisation were associated with different profiles of eczema, rhinitis, and wheeze. The main limitation of this study was the difference in wording of the questions used to ascertain the presence of eczema, wheeze, and rhinitis in the two cohorts.
Conclusions: The developmental profiles of eczema, wheeze, and rhinitis are heterogeneous; only a small proportion of children (∼ 7% of those with symptoms) follow trajectory profiles resembling the atopic march. Please see later in the article for the Editors' Summary.
Conflict of interest statement
AS has received travel grants and honoraria from GSK and Chiesi. She has received research grants from the Medical Research Council, National Institute of Health Research, and the JP Moulton Charitable Foundation. AC served as a consultant for Circassia. He received speaker fees from Glaxo Smith Kline, Thermo Fisher Scientific, Airsonet, Novartis, MSD, and ALK. He received research grants from the UK Medical Research Council, Moulton Charitable Foundation National Institute of Health Research. AC is a member of the Editorial Board of
Figures
References
-
- Wahn U (2000) What drives the allergic march? Allergy 55: 591–599. - PubMed
-
- Wahn U, Bergmann R, Kulig M, Forster J, Bauer CP (1997) The natural course of sensitisation and atopic disease in infancy and childhood. Pediatr Allergy Immunol 8: 16–20. - PubMed
-
- Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, et al. (2014) Atopic dermatitis and the atopic march revisited. Allergy 69: 17–27. - PubMed
-
- Burgess JA, Lowe AJ, Matheson MC, Varigos G, Abramson MJ, et al. (2009) Does eczema lead to asthma? J Asthma 46: 429–436. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

