Effectiveness of cognitive behavioural therapy augmentation in major depression treatment (ECAM study): study protocol for a randomised clinical trial
- PMID: 25335963
- PMCID: PMC4208050
- DOI: 10.1136/bmjopen-2014-006359
Effectiveness of cognitive behavioural therapy augmentation in major depression treatment (ECAM study): study protocol for a randomised clinical trial
Abstract
Introduction: Major depression is a serious mental disorder that causes substantial distress and impairment in individuals and places an enormous burden on society. Although antidepressant treatment is the most common therapy provided in routine practice, there is little evidence to guide second-line therapy for patients who have failed to respond to antidepressants. The aim of this paper is to describe the study protocol for a randomised controlled trial that measures the clinical effectiveness of cognitive behavioural therapy (CBT) as an augmentation strategy to treat patients with non-psychotic major depression identified as suboptimal responders to usual depression care.
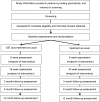
Methods and analysis: The current study is a 16-week assessor-blinded randomised, parallel-groups superiority trial with 12-month follow-up at an outpatient clinic as part of usual depression care. Patients aged 20-65 years with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Major Depressive Disorder who have experienced at least one failed trial of antidepressants as part of usual depression care, will be randomly assigned to receive CBT plus treatment as usual, or treatment as usual alone. The primary outcome is the change in clinician-rated 17-item GRID-Hamilton Depression Rating Scale (GRID-HAMD) score at 16 weeks, and secondary outcomes include severity and change in scores of subjective depression symptoms, proportion of responders and remitters, safety and quality of life. The primary population will be the intention-to-treat patients.
Ethics and dissemination: All protocols and the informed consent form comply with the Ethics Guideline for Clinical Research (Japanese Ministry of Health, Labour and Welfare). Ethics review committees at the Keio University School of Medicine and the Sakuragaoka Memorial Hospital approved the study protocol. The results of the study will be disseminated at several research conferences and as published articles in peer-reviewed journals. The study will be implemented and reported in line with the CONSORT statement.
Trial registration number: UMIN Clinical Trials Registry: UMIN000001218.
Keywords: clinical protocols; cognitive behavior therapy; major depressive disorder; randomized controlled trial.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Similar articles
-
Neural and clinical changes of cognitive behavioural therapy versus talking control in patients with major depression: a study protocol for a randomised clinical trial.BMJ Open. 2020 Feb 25;10(2):e029735. doi: 10.1136/bmjopen-2019-029735. BMJ Open. 2020. PMID: 32102803 Free PMC article.
-
Web-Based Cognitive Behavioral Therapy Blended With Face-to-Face Sessions for Major Depression: Randomized Controlled Trial.J Med Internet Res. 2018 Sep 21;20(9):e10743. doi: 10.2196/10743. J Med Internet Res. 2018. PMID: 30249583 Free PMC article. Clinical Trial.
-
Study protocol of the Diabetes and Depression Study (DAD): a multi-center randomized controlled trial to compare the efficacy of a diabetes-specific cognitive behavioral group therapy versus sertraline in patients with major depression and poorly controlled diabetes mellitus.BMC Psychiatry. 2013 Aug 6;13:206. doi: 10.1186/1471-244X-13-206. BMC Psychiatry. 2013. PMID: 23915015 Free PMC article. Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Assessing the efficacy of metacognitive therapy as monotherapy or adjunctive treatment in patient suffering from major depression and dysthimia: A comprehensive review of clinical trials.J Affect Disord. 2025 Feb 15;371:333-343. doi: 10.1016/j.jad.2024.11.050. Epub 2024 Nov 16. J Affect Disord. 2025. PMID: 39557303 Review.
Cited by
-
The Depression Inventory Development Workgroup: A Collaborative, Empirically Driven Initiative to Develop a New Assessment Tool for Major Depressive Disorder.Innov Clin Neurosci. 2016 Oct 1;13(9-10):20-31. eCollection 2016 Sep-Oct. Innov Clin Neurosci. 2016. PMID: 27974997 Free PMC article. Review.
-
Cost-effectiveness analyses of augmented cognitive behavioral therapy for pharmacotherapy-resistant depression at secondary mental health care settings.Psychiatry Clin Neurosci. 2021 Nov;75(11):341-350. doi: 10.1111/pcn.13298. Epub 2021 Sep 17. Psychiatry Clin Neurosci. 2021. PMID: 34459077 Free PMC article. Clinical Trial.
-
Psychological therapies for treatment-resistant depression in adults.Cochrane Database Syst Rev. 2018 May 14;5(5):CD010558. doi: 10.1002/14651858.CD010558.pub2. Cochrane Database Syst Rev. 2018. PMID: 29761488 Free PMC article.
-
The effect of cognitive behavioral therapy on future thinking in patients with major depressive disorder: A randomized controlled trial.Front Psychiatry. 2023 Jan 25;14:997154. doi: 10.3389/fpsyt.2023.997154. eCollection 2023. Front Psychiatry. 2023. PMID: 36761867 Free PMC article.
-
Cognitive behavioral therapy combined with "empathy nursing" model on recurrent depressive disorder.Am J Transl Res. 2021 Nov 15;13(11):12816-12824. eCollection 2021. Am J Transl Res. 2021. PMID: 34956496 Free PMC article.
References
-
- Hasin DS, Goodwin RD, Stinson FS, et al. . Epidemiology of major depressive disorder: Results from the national epidemiologic survey on alcoholism and related conditions. Arch Gen Psychiatry 2005;62:1097–106 - PubMed
-
- Lopez AD, Mathers CD, Ezzati M, et al. . Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–57 - PubMed
-
- Murray CJ, Vos T, Lozano R, et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical