Functionally important carboxyls in a bacterial homologue of the vesicular monoamine transporter (VMAT)
- PMID: 25336661
- PMCID: PMC4256354
- DOI: 10.1074/jbc.M114.607366
Functionally important carboxyls in a bacterial homologue of the vesicular monoamine transporter (VMAT)
Abstract
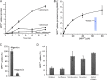
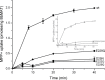
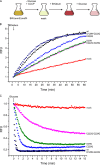
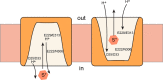
Transporters essential for neurotransmission in mammalian organisms and bacterial multidrug transporters involved in antibiotic resistance are evolutionarily related. To understand in more detail the evolutionary aspects of the transformation of a bacterial multidrug transporter to a mammalian neurotransporter and to learn about mechanisms in a milieu amenable for structural and biochemical studies, we identified, cloned, and partially characterized bacterial homologues of the rat vesicular monoamine transporter (rVMAT2). We performed preliminary biochemical characterization of one of them, Brevibacillus brevis monoamine transporter (BbMAT), from the bacterium B. brevis. BbMAT shares substrates with rVMAT2 and transports them in exchange with >1H(+), like the mammalian transporter. Here we present a homology model of BbMAT that has the standard major facilitator superfamily fold; that is, with two domains of six transmembrane helices each, related by 2-fold pseudosymmetry whose axis runs normal to the membrane and between the two halves. The model predicts that four carboxyl residues, a histidine, and an arginine are located in the transmembrane segments. We show here that two of the carboxyls are conserved, equivalent to the corresponding ones in rVMAT2, and are essential for H(+)-coupled transport. We conclude that BbMAT provides an excellent experimental paradigm for the study of its mammalian counterparts and bacterial multidrug transporters.
Keywords: Antibiotic Resistance; Membrane Transport; Multidrug Transporter; Neurotransmitter; Neurotransmitter Transport; Proton Transport.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Eiden L. E. (2000) The vesicular neurotransmitter transporters: current perspectives and future prospects. FASEB J. 14, 2396–2400 - PubMed
-
- Schuldiner S., Shirvan A., Linial M. (1995) Vesicular neurotransmitter transporters: from bacteria to humans. Physiol. Rev. 75, 369–392 - PubMed
-
- Chaudhry F. A., Edwards R. H., Fonnum F. (2008) Vesicular neurotransmitter transporters as targets for endogenous and exogenous toxic substances. Annu. Rev. Pharmacol. Toxicol. 48, 277–301 - PubMed
-
- Edwards R. (1992) The transport of neurotransmitters into synaptic vesicles. Curr. Opin. Neurobiol. 2, 586–594 - PubMed
-
- Yelin R., Schuldiner S. (1995) The pharmacological profile of the vesicular monoamine transporter resembles that of multidrug transporters. FEBS Lett. 377, 201–207 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

