Spatial gradients of protein-level time delays set the pace of the traveling segmentation clock waves
- PMID: 25336742
- PMCID: PMC4302894
- DOI: 10.1242/dev.111930
Spatial gradients of protein-level time delays set the pace of the traveling segmentation clock waves
Abstract
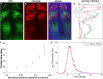
The vertebrate segmentation clock is a gene expression oscillator controlling rhythmic segmentation of the vertebral column during embryonic development. The period of oscillations becomes longer as cells are displaced along the posterior to anterior axis, which results in traveling waves of clock gene expression sweeping in the unsegmented tissue. Although various hypotheses necessitating the inclusion of additional regulatory genes into the core clock network at different spatial locations have been proposed, the mechanism underlying traveling waves has remained elusive. Here, we combined molecular-level computational modeling and quantitative experimentation to solve this puzzle. Our model predicts the existence of an increasing gradient of gene expression time delays along the posterior to anterior direction to recapitulate spatiotemporal profiles of the traveling segmentation clock waves in different genetic backgrounds in zebrafish. We validated this prediction by measuring an increased time delay of oscillatory Her1 protein production along the unsegmented tissue. Our results refuted the need for spatial expansion of the core feedback loop to explain the occurrence of traveling waves. Spatial regulation of gene expression time delays is a novel way of creating dynamic patterns; this is the first report demonstrating such a control mechanism in any tissue and future investigations will explore the presence of analogous examples in other biological systems.
Keywords: Computational modeling; Gene expression; Oscillation; Segmentation clock; Systems biology; Traveling wave.
© 2014. Published by The Company of Biologists Ltd.
Figures
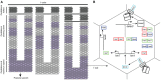

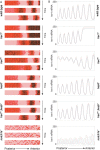
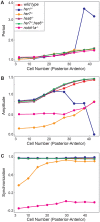
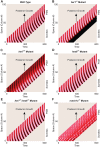
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

