G protein-coupled receptor 56 regulates mechanical overload-induced muscle hypertrophy
- PMID: 25336758
- PMCID: PMC4226111
- DOI: 10.1073/pnas.1417898111
G protein-coupled receptor 56 regulates mechanical overload-induced muscle hypertrophy
Abstract
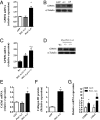
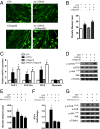
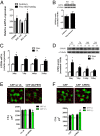
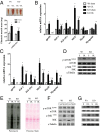
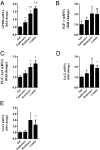
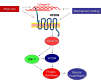
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha 4 (PGC-1α4) is a protein isoform derived by alternative splicing of the PGC1α mRNA and has been shown to promote muscle hypertrophy. We show here that G protein-coupled receptor 56 (GPR56) is a transcriptional target of PGC-1α4 and is induced in humans by resistance exercise. Furthermore, the anabolic effects of PGC-1α4 in cultured murine muscle cells are dependent on GPR56 signaling, because knockdown of GPR56 attenuates PGC-1α4-induced muscle hypertrophy in vitro. Forced expression of GPR56 results in myotube hypertrophy through the expression of insulin-like growth factor 1, which is dependent on Gα12/13 signaling. A murine model of overload-induced muscle hypertrophy is associated with increased expression of both GPR56 and its ligand collagen type III, whereas genetic ablation of GPR56 expression attenuates overload-induced muscle hypertrophy and associated anabolic signaling. These data illustrate a signaling pathway through GPR56 which regulates muscle hypertrophy associated with resistance/loading-type exercise.
Keywords: GPR56; Gα12/13; mTOR; muscle hypertrophy; overload.
Conflict of interest statement
Conflict of interest statement: D.J.G. and Z.W. are employees of, and stockholders in, Novartis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

