A DNA vaccine encoding a chimeric allergen derived from major group 1 allergens of dust mite can be used for specific immunotherapy
- PMID: 25337189
- PMCID: PMC4203160
A DNA vaccine encoding a chimeric allergen derived from major group 1 allergens of dust mite can be used for specific immunotherapy
Abstract
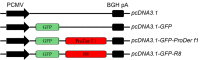
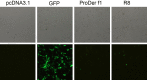
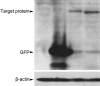
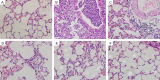
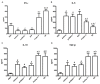
Immunization with DNA-based constructs has been shown to be against the antigen and the response is skewed in such a way as to ameliorate the symptoms of allergic disease. This approach is particularly useful in the treatment of allergic inflammatory diseases, such as asthma. The major group 1 allergen from house dust mites is one of the triggers of allergic asthma. This study explores whether a chimeric gene R8, derived from the major group 1 allergen of house dust mite species (Dermatophagoides farinae and Dermatophagoides pteronyssinus), can be expressed in Human Embryonic Kidney 293 cells (HEK 293 T) and whether such a construct can be used as a DNA vaccine in asthma therapy. The eukaryotic expression vector pcDNA3.1 was used to express the R8 molecule in HEK 293 T cells and successful expression of R8 was confirmed using a fluorescence microscope and western blot analysis. The efficacy of R8 as DNA vaccine was also assessed in a mouse asthma model. The in vivo data showed that R8 rectified the TH1/TH2 imbalance typical of allergic inflammation and stimulated the proliferation of regulatory T (Treg) cells. Immunization with the R8 construct also decreased serum allergen-specific IgE production in this mouse asthma model. Our findings suggest that R8 may be a feasible potential DNA vaccine for specific immunotherapy (SIT) in the treatment of allergic asthma.
Keywords: DNA vaccine; Dermatophagoides allergen 1 group; asthma; chimeric gene.
Figures

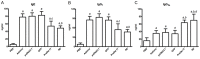
References
-
- Beasley R. The burden of asthma with specific reference to the United States. J Allergy Clin Immunol. 2002;109:S482–489. - PubMed
-
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. - PubMed
-
- Prescott SL. Maternal allergen exposure as a risk factor for childhood asthma. Curr Allergy Asthma Rep. 2006;6:75–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
