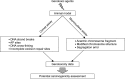
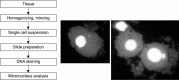
Recent advances in in vivo genotoxicity testing: prediction of carcinogenic potential using comet and micronucleus assay in animal models
- PMID: 25337557
- PMCID: PMC4189446
- DOI: 10.15430/jcp.2013.18.4.277
Recent advances in in vivo genotoxicity testing: prediction of carcinogenic potential using comet and micronucleus assay in animal models
Abstract
Genotoxic events have been known as crucial step in the initiation of cancer. To assess the risk of cancer, genotoxicity assays, including comet, micronucleus (MN), chromosomal aberration, bacterial reverse, and sister chromatid exchange assay, can be performed. Compared with in vitro genotoxicity assay, in vivo genotoxicity assay has been used to verify in vitro assay result and definitely provide biological significance for certain organs or cell types. The comet assay can detect DNA strand breaks as markers of genotoxicity. Methods of the in vivo comet assay have been established by Japanese Center for the Validation of Alternative Methods (JaCVAM) validation studies depending on tissue and sample types. The MN can be initiated by segregation error and lagging acentric chromosome fragment. Methods of the in vivo MN assay have been established by Organization for Economic Co-operation and Development (OECD) test guidelines and many studies. Combining the in vivo comet and MN assay has been regarded as useful methodology for evaluating genetic damage, and it has been used in the assessment of potential carcinogenicity by complementarily presenting two distinct endpoints of the in vivo genotoxicity individual test. Few studies have investigated the quantitative relation between in vivo genotoxicity results and carcinogenicity. Extensive studies emphasizes that positive correlation is detectable. This review summarizes the results of the in vivo comet and MN assays that have investigated the genotoxicity of carcinogens as classified by the International Agency for Research on Cancer (IARC) carcinogenicity database. As a result, these genotoxicity data may provide meaningful information for the assessment of potential carcinogenicity and for implementation in the prevention of cancer.
Keywords: Carcinogenicity; Comet assay; In vivo genotoxicity; Micronucleus assay.
Figures
Similar articles
-
The comet assay with multiple mouse organs: comparison of comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and U.S. NTP Carcinogenicity Database.Crit Rev Toxicol. 2000 Nov;30(6):629-799. doi: 10.1080/10408440008951123. Crit Rev Toxicol. 2000. PMID: 11145306 Review.
-
Results of the International Validation of the in vivo rodent alkaline comet assay for the detection of genotoxic carcinogens: Individual data for 1,2-dibromoethane, p-anisidine, and o-anthranilic acid in the 2nd step of the 4th phase Validation Study under the JaCVAM initiative.Mutat Res Genet Toxicol Environ Mutagen. 2015 Jul;786-788:144-50. doi: 10.1016/j.mrgentox.2015.03.002. Epub 2015 Mar 5. Mutat Res Genet Toxicol Environ Mutagen. 2015. PMID: 26212305
-
JaCVAM-organized international validation study of the in vivo rodent alkaline comet assay for detection of genotoxic carcinogens: II. Summary of definitive validation study results.Mutat Res Genet Toxicol Environ Mutagen. 2015 Jul;786-788:45-76. doi: 10.1016/j.mrgentox.2015.04.010. Epub 2015 Apr 29. Mutat Res Genet Toxicol Environ Mutagen. 2015. PMID: 26212295
-
In vivo genotoxicity testing strategies: Report from the 7th International workshop on genotoxicity testing (IWGT).Mutat Res Genet Toxicol Environ Mutagen. 2019 Nov;847:403035. doi: 10.1016/j.mrgentox.2019.03.008. Epub 2019 Apr 25. Mutat Res Genet Toxicol Environ Mutagen. 2019. PMID: 31699340
-
Combinations of genotoxic tests for the evaluation of group 1 IARC carcinogens.J Appl Toxicol. 2018 Jan;38(1):81-99. doi: 10.1002/jat.3496. Epub 2017 Jul 11. J Appl Toxicol. 2018. PMID: 28695982 Review.
Cited by
-
Characterization and assessment of the genotoxicity and biocompatibility of poly (hydroxybutyrate) and norbixin membranes.Acta Cir Bras. 2020;35(7):e202000706. doi: 10.1590/s0102-865020200070000006. Epub 2020 Aug 28. Acta Cir Bras. 2020. PMID: 32876084 Free PMC article.
-
Investigation of genotoxicity, mutagenicity, and cytotoxicity in erythrocytes of Nile tilapia (Oreochromis niloticus) after fluoxetine exposure.Toxicol Rep. 2022 Mar 29;9:588-596. doi: 10.1016/j.toxrep.2022.03.031. eCollection 2022. Toxicol Rep. 2022. PMID: 35392157 Free PMC article.
-
Induced cytotoxic damage by exposure to gasoline vapors: a study in Sinaloa, Mexico.Environ Sci Pollut Res Int. 2017 Jan;24(1):539-546. doi: 10.1007/s11356-016-7821-8. Epub 2016 Oct 12. Environ Sci Pollut Res Int. 2017. PMID: 27734313
-
A comprehensive review on clinical applications of comet assay.J Clin Diagn Res. 2015 Mar;9(3):GE01-5. doi: 10.7860/JCDR/2015/12062.5622. Epub 2015 Mar 1. J Clin Diagn Res. 2015. PMID: 25954633 Free PMC article. Review.
-
Pathways of Metabolite-Related Damage to a Synthetic p53 Gene Exon 7 Oligonucleotide Using Magnetic Enzyme Bioreactor Beads and LC-MS/MS Sequencing.Biochemistry. 2018 Jul 3;57(26):3883-3893. doi: 10.1021/acs.biochem.8b00271. Epub 2018 May 23. Biochemistry. 2018. PMID: 29750510 Free PMC article.
References
-
- Pratheepa Sivasankari N, Kaur S, Reddy KS, Vivekanandam S. Micronucleus Index: an early diagnosis in oral carcinoma. J Anat Soc India. 2008;57:8–13.
-
- Keen-Kim D, Nooraie F, Rao PN. Cytogenetic biomarkers for human cancer. Front Biosci. 2008;13:5928. - PubMed
-
- Miyamae Y, Yamamoto M, Sasaki YF, Kobayashi H, Igarashi-Soga M, Shimoi K, et al. Evaluation of a tissue homogenization technique that isolates nuclei for the in vivo single cell gel electrophoresis (comet) assay: a collaborative study by five laboratories. Mutat Res. 1998;418:131–40. - PubMed
-
- Brendler-Schwaab S, Hartmann A, Pfuhler S, Speit G. The in vivo comet assay: use and status in genotoxicity testing. Mutagenesis. 2005;20:245–54. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials