Steroid hormone intervenes in the endometrial tumorigenesis of pten ablation
- PMID: 25337560
- PMCID: PMC4189443
- DOI: 10.15430/jcp.2013.18.4.313
Steroid hormone intervenes in the endometrial tumorigenesis of pten ablation
Abstract
Background: Endometrial cancer, the most common gynecological cancer, is closely associated with endometrial hyperplasia, unopposed estrogen exposure, and genetic alterations. Phosphatase and tensin homologue (PTEN) is a tumor suppressor genes completely lost or mutated in >50% of primary endometrioid endometrial cancers. Estrogen-dependent endometrioid carcinoma is the most common type of endometrial cancer. Progesterone is a hormone that antagonizes the growth-promoting properties of estrogen in the uterus. Progestin is used as a conservative endocrine treatment of early endometrial cancer in order to preserve fertility as well as a palliative measure for advanced-stage patients. Progesterone therapy has been shown to be effective in preventing endometrial cancer as well as controlling growth of the endometrium. However, the effectiveness of progestin for women with endometrial cancer is less clear.
Methods: In order to understand the effect of steroid hormone on endometrial cancer progression, we used a mouse endometrial cancer model with conditional loss of Pten in the mouse uterus (PR (cre/+) Pten (f/f) , Pten(d/d) ). To assess the effect of steroid hormones, ovariectomized Pten(f/f) and Pten(d/d) mice were treated with estrogen or progesterone over a period of three month.
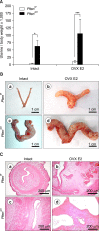
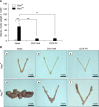
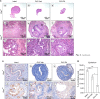
Results: Uterine weight gain was significantly decreased in ovariectomized PR (cre/+) Pten(f/f) mice compared to intact PR (cre/+) Pten(f/f) mice. Ovariectomized PR (cre/+) Pten(f/f) mice treated with P4 or vehicle also exhibited decreased uterine cancer size compared with intact PR (cre/+) Pten(f/f) mice. Proliferation of ovariectomized PR (cre/+) Pten(f/f) mice treated with P4 is highly decreased compared to other groups. The levels of stromal progesterone receptor were highly increased in ovariectomized PR (cre/+) Pten(f/f) mice treated with P4 which resulted in decreased epithelial proliferation.
Conclusions: These results suggest that P4 treatment significantly reduces tumor mass but does not affect cancer progression in PR (cre/+) Pten(f/f) mice.
Keywords: Endometrial cancer; Estrogen; PTEN; Progesterone; Progesterone receptor.
Figures

Similar articles
-
Critical tumor suppressor function mediated by epithelial Mig-6 in endometrial cancer.Cancer Res. 2013 Aug 15;73(16):5090-9. doi: 10.1158/0008-5472.CAN-13-0241. Epub 2013 Jun 27. Cancer Res. 2013. PMID: 23811943 Free PMC article.
-
The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression.Oncogene. 2010 Jul 1;29(26):3770-80. doi: 10.1038/onc.2010.126. Epub 2010 Apr 26. Oncogene. 2010. PMID: 20418913 Free PMC article.
-
MIG-6 suppresses endometrial epithelial cell proliferation by inhibiting phospho-AKT.BMC Cancer. 2018 May 29;18(1):605. doi: 10.1186/s12885-018-4502-7. BMC Cancer. 2018. PMID: 29843645 Free PMC article.
-
[Endocrinological contribution for invasion and metastasis in gynecological cancers].Nihon Sanka Fujinka Gakkai Zasshi. 1996 Aug;48(8):633-43. Nihon Sanka Fujinka Gakkai Zasshi. 1996. PMID: 8808831 Review. Japanese.
-
Biology of progesterone action during pregnancy recognition and maintenance of pregnancy.Front Biosci. 2002 Sep 1;7:d1879-98. doi: 10.2741/spencer. Front Biosci. 2002. PMID: 12161340 Review.
Cited by
-
ERBB2 Targeting Reveals a Significant Suppression of Tumorigenesis in Murine Endometrial Cancer with Pten Mutation.Reprod Sci. 2024 Aug;31(8):2458-2467. doi: 10.1007/s43032-024-01546-3. Epub 2024 Apr 18. Reprod Sci. 2024. PMID: 38637476
-
The interplay of obesity, microbiome dynamics, and innovative anti-obesity strategies in the context of endometrial cancer progression and therapeutic approaches.Biochim Biophys Acta Rev Cancer. 2023 Nov;1878(6):189000. doi: 10.1016/j.bbcan.2023.189000. Epub 2023 Oct 14. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37844671 Free PMC article. Review.
-
FOXP1 forkhead transcription factor is associated with the pathogenesis of endometrial cancer.Heliyon. 2016 May 27;2(5):e00116. doi: 10.1016/j.heliyon.2016.e00116. eCollection 2016 May. Heliyon. 2016. PMID: 27441287 Free PMC article.
-
Endometrial biomarkers in premenopausal women with obesity: an at-risk cohort.Am J Obstet Gynecol. 2021 Mar;224(3):278.e1-278.e14. doi: 10.1016/j.ajog.2020.08.053. Epub 2020 Aug 21. Am J Obstet Gynecol. 2021. PMID: 32835719 Free PMC article.
-
MIG-6 Is Critical for Progesterone Responsiveness in Human Complex Atypical Hyperplasia and Early-Stage Endometrial Cancer.Int J Mol Sci. 2022 Nov 23;23(23):14596. doi: 10.3390/ijms232314596. Int J Mol Sci. 2022. PMID: 36498921 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Jick SS. Combined estrogen and progesterone use and endometrial cancer. Epidemiology. 1993;4:384. - PubMed
-
- Jick SS, Walker AM, Jick H. Estrogens, progesterone, and endometrial cancer. Epidemiology. 1993;4:20–4. - PubMed
-
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. - PubMed
-
- Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–54. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
