High-dose rifapentine with moxifloxacin for pulmonary tuberculosis
- PMID: 25337749
- PMCID: PMC4233406
- DOI: 10.1056/NEJMoa1314210
High-dose rifapentine with moxifloxacin for pulmonary tuberculosis
Abstract
Background: Tuberculosis regimens that are shorter and simpler than the current 6-month daily regimen are needed.
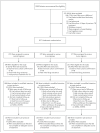
Methods: We randomly assigned patients with newly diagnosed, smear-positive, drug-sensitive tuberculosis to one of three regimens: a control regimen that included 2 months of ethambutol, isoniazid, rifampicin, and pyrazinamide administered daily followed by 4 months of daily isoniazid and rifampicin; a 4-month regimen in which the isoniazid in the control regimen was replaced by moxifloxacin administered daily for 2 months followed by moxifloxacin and 900 mg of rifapentine administered twice weekly for 2 months; or a 6-month regimen in which isoniazid was replaced by daily moxifloxacin for 2 months followed by one weekly dose of both moxifloxacin and 1200 mg of rifapentine for 4 months. Sputum specimens were examined on microscopy and after culture at regular intervals. The primary end point was a composite treatment failure and relapse, with noninferiority based on a margin of 6 percentage points and 90% confidence intervals.
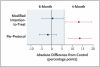
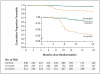
Results: We enrolled a total of 827 patients from South Africa, Zimbabwe, Botswana, and Zambia; 28% of patients were coinfected with the human immunodefiency virus. In the per-protocol analysis, the proportion of patients with an unfavorable response was 4.9% in the control group, 3.2% in the 6-month group (adjusted difference from control, -1.8 percentage points; 90% confidence interval [CI], -6.1 to 2.4), and 18.2% in the 4-month group (adjusted difference from control, 13.6 percentage points; 90% CI, 8.1 to 19.1). In the modified intention-to-treat analysis these proportions were 14.4% in the control group, 13.7% in the 6-month group (adjusted difference from control, 0.4 percentage points; 90% CI, -4.7 to 5.6), and 26.9% in the 4-month group (adjusted difference from control, 13.1 percentage points; 90% CI, 6.8 to 19.4).
Conclusions: The 6-month regimen that included weekly administration of high-dose rifapentine and moxifloxacin was as effective as the control regimen. The 4-month regimen was not noninferior to the control regimen. (Funded by the European and Developing Countries Clinical Trials Partnership and the Wellcome Trust; RIFAQUIN Current Controlled Trials number, ISRCTN44153044.).
Figures
Comment in
-
Shortening treatment for tuberculosis--to basics.N Engl J Med. 2014 Oct 23;371(17):1642-3. doi: 10.1056/NEJMe1410977. N Engl J Med. 2014. PMID: 25337754 No abstract available.
References
-
- Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet. 2004;364:1244–51. - PubMed
-
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946-1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(Suppl 2):S231–79. - PubMed
-
- Food and Drug Administration Priftin (rifapentine) drug approval package. 2000 Oct; http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21024S5_Priftin.cfm.
-
- Tam CM, Chan SL, Lam CW, et al. Rifapentine and isoniazid in the continuation phase of treating pulmonary tuberculosis; initial report. Am J Respir Crit Care Med. 1998;157:1726–33. - PubMed
-
- Benator D, Bhattacharya M, Bozeman L, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360:528–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
