Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism
- PMID: 25337774
- PMCID: PMC4382689
- DOI: 10.1038/ki.2014.349
Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism
Abstract
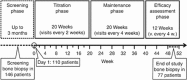
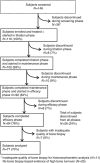
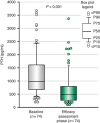
The multicenter, single-arm BONAFIDE study characterized the skeletal response to cinacalcet in adult dialysis patients with plasma parathyroid hormone (PTH) levels of 300 pg/ml or more, serum calcium of 8.4 mg/dl or more, bone-specific alkaline phosphatase over 20.9 ng/ml and biopsy-proven high-turnover bone disease. Of 110 enrolled patients, 77 underwent a second bone biopsy with quantitative histomorphometry after 6-12 months of cinacalcet treatment. The median PTH decreased from 985 pg/ml at baseline to 480 pg/ml at the end of study (weeks 44-52). Bone formation rate/tissue area decreased from 728 to 336 μm(2)/mm(2)/day, osteoblast perimeter/osteoid perimeter decreased from 17.4 to 13.9%, and eroded perimeter/bone perimeter decreased from 12.7 to 8.3%. The number of patients with normal bone histology increased from none at baseline to 20 at 12 months. Two patients had adynamic bone at the end of study with a PTH under 150 pg/ml, and one patient with overt hypophosphatemia at baseline that reoccurred during follow-up developed osteomalacia. Thus, long-term treatment with cinacalcet substantially reduced PTH, diminished the elevated bone formation rate/tissue area, lowered several biochemical markers of high-turnover bone disease toward normal, and generally improved bone histology. Twenty patients had normal bone histology at follow-up, whereas most had mild hyperparathyroidism or mixed uremic osteodystrophy.
Figures


References
-
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group KDIGO Clinical practice guidelines for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76 (Suppl 113:S1–S130. - PubMed
-
- Goodman WG, Hladik GA, Turner SA, et al. The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol. 2002;13:1017–1024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

