A set of computationally designed orthogonal antiparallel homodimers that expands the synthetic coiled-coil toolkit
- PMID: 25337788
- PMCID: PMC4277747
- DOI: 10.1021/ja507847t
A set of computationally designed orthogonal antiparallel homodimers that expands the synthetic coiled-coil toolkit
Abstract
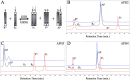
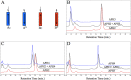
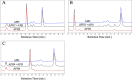
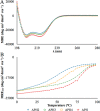
Molecular engineering of protein assemblies, including the fabrication of nanostructures and synthetic signaling pathways, relies on the availability of modular parts that can be combined to give different structures and functions. Currently, a limited number of well-characterized protein interaction components are available. Coiled-coil interaction modules have been demonstrated to be useful for biomolecular design, and many parallel homodimers and heterodimers are available in the coiled-coil toolkit. In this work, we sought to design a set of orthogonal antiparallel homodimeric coiled coils using a computational approach. There are very few antiparallel homodimers described in the literature, and none have been measured for cross-reactivity. We tested the ability of the distance-dependent statistical potential DFIRE to predict orientation preferences for coiled-coil dimers of known structure. The DFIRE model was then combined with the CLASSY multistate protein design framework to engineer sets of three orthogonal antiparallel homodimeric coiled coils. Experimental measurements confirmed the successful design of three peptides that preferentially formed antiparallel homodimers that, furthermore, did not interact with one additional previously reported antiparallel homodimer. Two designed peptides that formed higher-order structures suggest how future design protocols could be improved. The successful designs represent a significant expansion of the existing protein-interaction toolbox for molecular engineers.
Figures


References
-
- Purnick P. E.; Weiss R. Nat. Rev. Mol. Cell Biol. 2009, 10, 410–422. - PubMed
-
- Fletcher J. M.; Boyle A. L.; Bruning M.; Bartlett G. J.; Vincent T. L.; Zacci N. R.; Armstrong C. T.; Bromley E. H. C.; Booth P. J.; Brady R. L.; Thomson A. R.; Woolfson D. N. ACS Synth. Biol. 2012, 1, 240–250. - PubMed
-
- Rackham O. J.; Madera M.; Armstrong C. T.; Vincent T. L.; Woolfson D. N.; Gough J. J. Mol. Biol. 2010, 403, 480–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

