Identification of MMV Malaria Box inhibitors of Perkinsus marinus using an ATP-based bioluminescence assay
- PMID: 25337810
- PMCID: PMC4206467
- DOI: 10.1371/journal.pone.0111051
Identification of MMV Malaria Box inhibitors of Perkinsus marinus using an ATP-based bioluminescence assay
Abstract
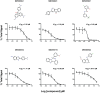
"Dermo" disease caused by the protozoan parasite Perkinsus marinus (Perkinsozoa) is one of the main obstacles to the restoration of oyster populations in the USA. Perkinsus spp. are also a concern worldwide because there are limited approaches to intervention against the disease. Based on the phylogenetic affinity between the Perkinsozoa and Apicomplexa, we exposed Perkinsus trophozoites to the Medicines for Malaria Venture Malaria Box, an open access compound library comprised of 200 drug-like and 200 probe-like compounds that are highly active against the erythrocyte stage of Plasmodium falciparum. Using a final concentration of 20 µM, we found that 4 days after exposure 46% of the compounds were active against P. marinus trophozoites. Six compounds with IC50 in the µM range were used to compare the degree of susceptibility in vitro of eight P. marinus strains from the USA and five Perkinsus species from around the world. The three compounds, MMV666021, MMV665807 and MMV666102, displayed a uniform effect across Perkinsus strains and species. Both Perkinsus marinus isolates and Perkinsus spp. presented different patterns of response to the panel of compounds tested, supporting the concept of strain/species variability. Here, we expanded the range of compounds available for inhibiting Perkinsus proliferation in vitro and characterized Perkinsus phenotypes based on their resistance to six compounds. We also discuss the implications of these findings in the context of oyster management. The Perkinsus system offers the potential for investigating the mechanism of action of the compounds of interest.
Conflict of interest statement
Figures

References
-
- Mackin JG, Owen HM, Collier A (1950) Preliminary note on the occurrence of a new protistan parasite, Dermocystidium marinum n. sp., in Crassostrea virginica (Gmelin). Science 111: 328–329. - PubMed
-
- Ford SE, Chintala MM (2006) Northward expansion of a marine parasite: Testing the role of temperature adaptation. J Exp Mar Bio Ecol 339: 226–235.
-
- Ford SE, Smolowitz R (2007) Infection dynamics of an oyster parasite in its newly expanded range. Mar Biol 151: 119–133.
-
- Smolowitz R (2013) A review of current state of knowledge concerning Perkinsus marinus effects on Crassostrea virginica (Gmelin) (the eastern oyster). Vet Pathol 50: 404–411. - PubMed
-
- Pecher WT, Alavi MR, Schott EJ, Fernández-Robledo JA, Roth L, et al. (2008) Assessment of the northern distribution range of selected Perkinsus species in eastern oysters (Crassostrea virginica) and hard clams (Mercenaria mercenaria) with the use of PCR-based detection assays. J Parasitol 94: 410–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

