An increase in CD3+CD4+CD25+ regulatory T cells after administration of umbilical cord-derived mesenchymal stem cells during sepsis
- PMID: 25337817
- PMCID: PMC4206342
- DOI: 10.1371/journal.pone.0110338
An increase in CD3+CD4+CD25+ regulatory T cells after administration of umbilical cord-derived mesenchymal stem cells during sepsis
Abstract
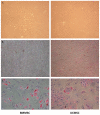
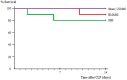
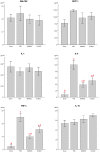
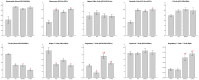
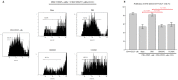
Sepsis remains an important cause of death worldwide, and vigorous immune responses during sepsis could be beneficial for bacterial clearance but at the price of collateral damage to self tissues. Mesenchymal stem cells (MSCs) have been found to modulate the immune system and attenuate sepsis. In the present study, MSCs derived from bone marrow and umbilical cord were used and compared. With a cecal ligation and puncture (CLP) model, the mechanisms of MSC-mediated immunoregulation during sepsis were studied by determining the changes of circulating inflammation-associated cytokine profiles and peripheral blood mononuclear cells 18 hours after CLP-induced sepsis. In vitro, bone marrow-derived MSCs (BMMSCs) and umbilical cord-derived MSCs (UCMSCs) showed a similar morphology and surface marker expression. UCMSCs had stronger potential for osteogenesis but lower for adipogenesis than BMMSCs. Compared with rats receiving PBS only after CLP, the percentage of circulating CD3+CD4+CD25+ regulatory T (Treg) cells and the ratio of Treg cells/T cells were elevated significantly in rats receiving MSCs. Further experiment regarding Treg cell function demonstrated that the immunosuppressive capacity of Treg cells from rats with CLP-induced sepsis was decreased, but could be restored by administration of MSCs. Compared with rats receiving PBS only after CLP, serum levels of interleukin-6 and tumor necrosis factor-α were significantly lower in rats receiving MSCs after CLP. There were no differences between BMMSCs and UCMSCs. In summary, this work provides the first in vivo evidence that administering BMMSCs or UCMSCs to rats with CLP-induced sepsis could increase circulating CD3+CD4+CD25+ Treg cells and Treg cells/T cells ratio, enhance Treg cell suppressive function, and decrease serum levels of interleukin-6 and tumor necrosis factor-α, suggesting the immunomodulatory association of Treg cells and MSCs during sepsis.
Conflict of interest statement
Figures

References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310. - PubMed
-
- Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213: 341–347. - PubMed
-
- Nauta AJ, Fibbe WE (2007) Immunomodulatory properties of mesenchymal stromal cells. Blood 110: 3499–3506. - PubMed
-
- English K (2013) Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol 91: 19–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

