Structure and insights into the function of a Ca(2+)-activated Cl(-) channel
- PMID: 25337878
- PMCID: PMC4454446
- DOI: 10.1038/nature13913
Structure and insights into the function of a Ca(2+)-activated Cl(-) channel
Abstract
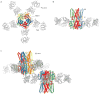
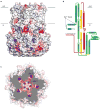
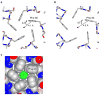
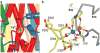
Bestrophin calcium-activated chloride channels (CaCCs) regulate the flow of chloride and other monovalent anions across cellular membranes in response to intracellular calcium (Ca(2+)) levels. Mutations in bestrophin 1 (BEST1) cause certain eye diseases. Here we present X-ray structures of chicken BEST1-Fab complexes, at 2.85 Å resolution, with permeant anions and Ca(2+). Representing, to our knowledge, the first structure of a CaCC, the eukaryotic BEST1 channel, which recapitulates CaCC function in liposomes, is formed from a pentameric assembly of subunits. Ca(2+) binds to the channel's large cytosolic region. A single ion pore, approximately 95 Å in length, is located along the central axis and contains at least 15 binding sites for anions. A hydrophobic neck within the pore probably forms the gate. Phenylalanine residues within it may coordinate permeating anions via anion-π interactions. Conformational changes observed near the 'Ca(2+) clasp' hint at the mechanism of Ca(2+)-dependent gating. Disease-causing mutations are prevalent within the gating apparatus.
Conflict of interest statement
Figures







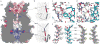
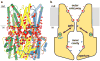
References
-
- Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annual review of physiology. 2005;67:719–758. - PubMed
-
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJV. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science (New York, NY) 2008;322:590–594. - PubMed
-
- Yang Y, Cho H, Koo J, Tak M, Cho Y, Shim W, Park S, Lee J, Lee B, Kim B, Raouf R, Shin Y, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

