Loss of HIF-1α in the notochord results in cell death and complete disappearance of the nucleus pulposus
- PMID: 25338007
- PMCID: PMC4206488
- DOI: 10.1371/journal.pone.0110768
Loss of HIF-1α in the notochord results in cell death and complete disappearance of the nucleus pulposus
Abstract
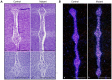
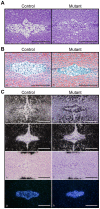
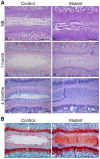
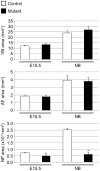
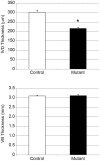
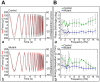
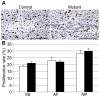
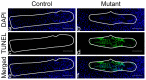
The intervertebral disc (IVD) is one of the largest avascular organs in vertebrates. The nucleus pulposus (NP), a highly hydrated and proteoglycan-enriched tissue, forms the inner portion of the IVD. The NP is surrounded by a multi-lamellar fibrocartilaginous structure, the annulus fibrosus (AF). This structure is covered superior and inferior side by cartilaginous endplates (CEP). The NP is a unique tissue within the IVD as it results from the differentiation of notochordal cells, whereas, AF and CEP derive from the sclerotome. The hypoxia inducible factor-1α (HIF-1α) is expressed in NP cells but its function in NP development and homeostasis is largely unknown. We thus conditionally deleted HIF-1α in notochordal cells and investigated how loss of this transcription factor impacts NP formation and homeostasis at E15.5, birth, 1 and 4 months of age, respectively. Histological analysis, cell lineage studies, and TUNEL assay were performed. Morphologic changes of the mutant NP cells were identified as early as E15.5, followed, postnatally, by the progressive disappearance and replacement of the NP with a novel tissue that resembles fibrocartilage. Notably, lineage studies and TUNEL assay unequivocally proved that NP cells did not transdifferentiate into chondrocyte-like cells but they rather underwent massive cell death, and were completely replaced by a cell population belonging to a lineage distinct from the notochordal one. Finally, to evaluate the functional consequences of HIF-1α deletion in the NP, biomechanical testing of mutant IVD was performed. Loss of the NP in mutant mice significantly reduced the IVD biomechanical properties by decreasing its ability to absorb mechanical stress. These findings are similar to the changes usually observed during human IVD degeneration. Our study thus demonstrates that HIF-1α is essential for NP development and homeostasis, and it raises the intriguing possibility that this transcription factor could be involved in IVD degeneration in humans.
Conflict of interest statement
Figures


References
-
- Raj PP (2008) Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain practice: the official journal of World Institute of Pain 8: 18–44. - PubMed
-
- Melrose J, Smith SM, Appleyard RC, Little CB (2008) Aggrecan, versican and type VI collagen are components of annular translamellar crossbridges in the intervertebral disc. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 17: 314–324. - PMC - PubMed
-
- Moon SM, Yoder JH, Wright AC, Smith LJ, Vresilovic EJ, et al. (2013) Evaluation of intervertebral disc cartilaginous endplate structure using magnetic resonance imaging. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 22: 1820–1828. - PMC - PubMed
-
- Choi KS, Cohn MJ, Harfe BD (2008) Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Developmental dynamics: an official publication of the American Association of Anatomists 237: 3953–3958. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

