Dextramer reagents are effective tools for quantifying CMV antigen-specific T cells from peripheral blood samples
- PMID: 25338522
- PMCID: PMC4376327
- DOI: 10.1002/cyto.b.21196
Dextramer reagents are effective tools for quantifying CMV antigen-specific T cells from peripheral blood samples
Abstract
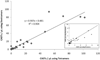
The enumeration of antigen-specific T cells is increasingly relevant in clinical and research settings. This information is useful for evaluating immune responses to treatment, monitoring the efficacy of anticancer vaccines, and for detecting self-reactive T cells in autoimmune disorders. Quantifying antigen-specific T cells can be accomplished via IFNγ ELISpot assay, the measurement of intracellular cytokine production by flow cytometry, or by lymphocyte proliferation assays in response to antigen. While robust, these technologies are labor-intensive and can take several days to obtain results. New technology has led to more powerful tools for quickly and accurately measuring antigen-specific T cells by flow cytometry via fluorescently-labeled TCR-specific multimers. In this study, we evaluated the use of an assay based on Dextramer reagents for enumerating cytomegalovirus (CMV) antigen-specific T cells (CASTs). Assay performance characteristics were assessed by establishing Dextramers' sensitivity (median=0.4; range=0.1-1.4 CASTs μl(-1) ), determining their specificity (100%), evaluating assay robustness with different leukocyte sources and assay reproducibility via interlaboratory and interinstrument investigations. Furthermore, the levels of CASTs in 95 peripheral blood samples from 62 unique blood and marrow transplants recipients correlated well between Dextramers and Tetramers (R(2) =0.9042).
Keywords: Dextramers; antigen-specific T-cell; cytomegalovirus; flow cytometry; immune monitoring.
© 2014 Clinical Cytometry Society.
Figures





References
-
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. - PubMed
-
- Matsui K, Boniface JJ, Reay PA, Schild H, Fazekas de St Groth B, Davis MM. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991;254:1788–1791. - PubMed
-
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. - PubMed
-
- Bakker AH, Schumacher TN. MHC multimer technology: current status and future prospects. Curr Opin Immunol. 2005;17:428–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

