Early engineering approaches to improve peptide developability and manufacturability
- PMID: 25338742
- PMCID: PMC4287292
- DOI: 10.1208/s12248-014-9681-9
Early engineering approaches to improve peptide developability and manufacturability
Abstract
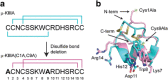
Downstream success in Pharmaceutical Development requires thoughtful molecule design early in the lifetime of any potential therapeutic. Most therapeutic monoclonal antibodies are quite similar with respect to their developability properties. However, the properties of therapeutic peptides tend to be as diverse as the molecules themselves. Analysis of the primary sequence reveals sites of potential adverse posttranslational modifications including asparagine deamidation, aspartic acid isomerization, methionine, tryptophan, and cysteine oxidation and, potentially, chemical and proteolytic degradation liabilities that can impact the developability and manufacturability of a potential therapeutic peptide. Assessing these liabilities, both biophysically and functionally, early in a molecule's lifetime can drive a more effective path forward in the drug discovery process. In addition to these potential liabilities, more complex peptides that contain multiple disulfide bonds can pose particular challenges with respect to production and manufacturability. Approaches to reducing the disulfide bond complexity of these peptides are often explored with mixed success. Proteolytic degradation is a major contributor to decreased half-life and efficacy. Addressing this aspect of peptide stability early in the discovery process increases downstream success. We will address aspects of peptide sequence analysis, molecule complexity, developability analysis, and manufacturing routes that drive the decision making processes during peptide therapeutic development.
Figures

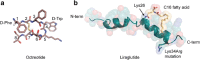
Comment in
-
Peptide Developability at the Discovery-to-Development Interface--Current State and Future Opportunities.AAPS J. 2015 Jul;17(4):777-9. doi: 10.1208/s12248-015-9755-3. Epub 2015 Mar 31. AAPS J. 2015. PMID: 25823670 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

