Two-pore domain potassium channels in the adrenal cortex
- PMID: 25339223
- PMCID: PMC4428839
- DOI: 10.1007/s00424-014-1628-6
Two-pore domain potassium channels in the adrenal cortex
Abstract
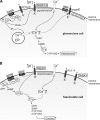
The physiological control of steroid hormone secretion from the adrenal cortex depends on the function of potassium channels. The "two-pore domain K(+) channels" (K2P) TWIK-related acid sensitive K(+) channel 1 (TASK1), TASK3, and TWIK-related K(+) channel 1 (TREK1) are strongly expressed in adrenocortical cells. They confer a background K(+) conductance to these cells which is important for the K(+) sensitivity as well as for angiotensin II and adrenocorticotropic hormone-dependent stimulation of aldosterone and cortisol synthesis. Mice with single deletions of the Task1 or Task3 gene as well as Task1/Task3 double knockout mice display partially autonomous aldosterone synthesis. It appears that TASK1 and TASK3 serve different functions: TASK1 affects cell differentiation and prevents expression of aldosterone synthase in the zona fasciculata, while TASK3 controls aldosterone secretion in glomerulosa cells. TREK1 is involved in the regulation of cortisol secretion in fasciculata cells. These data suggest that a disturbed function of K2P channels could contribute to adrenocortical pathologies in humans.
Figures
References
-
- Akizuki O, Inayoshi A, Kitayama T, Yao K, Shirakura S, Sasaki K, Kusaka H, Matsubara M. Blockade of T-type voltage-dependent Ca2+ channels by benidipine, a dihydropyridine calcium channel blocker, inhibits aldosterone production in human adrenocortical cell line NCI-H295R. Eur J Pharmacol. 2008;584:424–434. - PubMed
-
- Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–E829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

