Myofiber HLA-DR expression is a distinctive biomarker for antisynthetase-associated myopathy
- PMID: 25339355
- PMCID: PMC4210467
- DOI: 10.1186/s40478-014-0154-2
Myofiber HLA-DR expression is a distinctive biomarker for antisynthetase-associated myopathy
Abstract
Objectives: To assess the value of major histocompatibility complex (MHC) class II antigen (HLA-DR) expression to distinguish anti-synthetase myopathy (ASM) from dermatomyositis (DM).
Methods: Muscle biopsies from patients with ASM (n = 33), DM without anti-synthetase antibodies (ASAb) (n = 17), and normal muscle biopsy (n = 10) were first reviewed. ASAb included anti-Jo1 (26/33), anti-PL12 (4/33), anti-PL7 (2/33), and anti-EJ (1/33). Immunohistochemistry was performed for MHC-I/HLA-ABC, MHC-II/HLA-DR, membrane attack complex (C5b-9), neural cell adhesion molecule (NCAM)/CD56 expression, and inflammatory cell subsets. Twenty-four ASM and 12 DM patients from another center were added for HLA-DR evaluation.
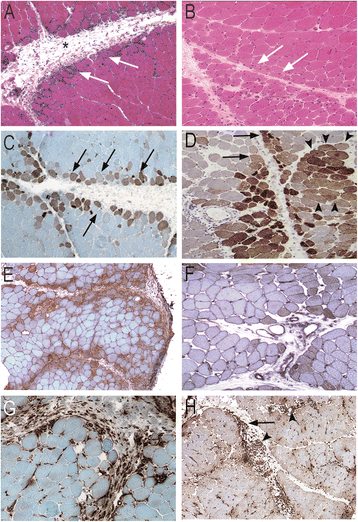
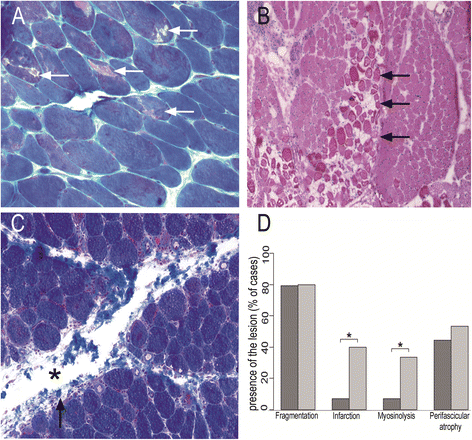
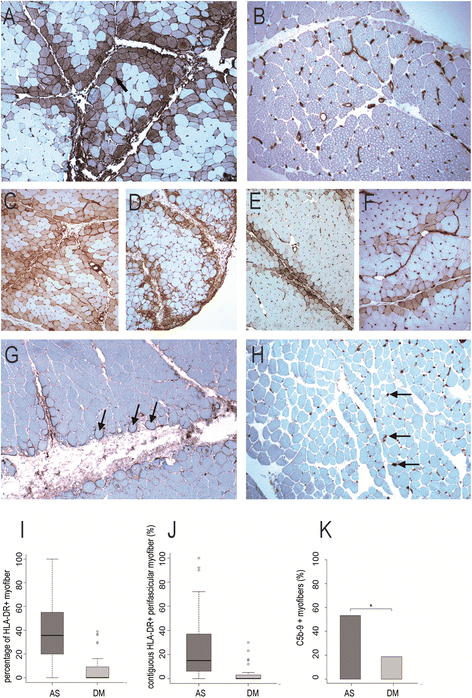
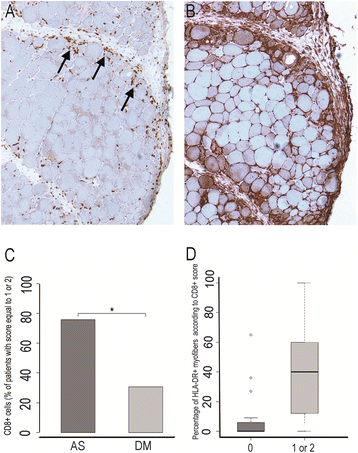
Results: Ubiquitous myofiber HLA-ABC expression was equally observed in ASM and DM (93.9% vs 100%, NS). In contrast, myofiber HLA-DR expression was found in 27/33 (81.8%) ASM (anti-Jo1: 23/26, 88.5%; others: 5/7, 71.4%) vs 4/17 (23.5%) DM patients (p < 0.001). HLA-DR was perifascicular in ASM, a pattern not observed in DM. In addition, C5b-9 deposition was observed on sarcolemma of non-necrotic perifascicular fibers in ASM, while, in DM, C5b-9was mainly detected in endomysial capillaries. CD8 cells were more abundant in ASM than in DM (p < 0.05), and electively located in perimysium or in perifascular endomysium. HLA-DR expression correlated positively with the CD8+ cells infiltrates. Strictly similar observations were made in the confirmatory study.
Conclusion: ASM is characterized by strong myofiber MHC-II/HLA-DR expression with a unique perifascicular pattern, not described so far. HLA-DR detection must be included for routine myopathological diagnosis of inflammatory/dysimmune myopathies. HLA-DR expression in ASM may indicate a specific immune mechanism, possibly involving IFNγ.
Figures
References
-
- Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, Vencovsky J, de Visser M, Hughes RA. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14(5):337–345. doi: 10.1016/j.nmd.2004.02.006. - DOI - PubMed
-
- Ranque B, Bérezné A, Le-Guern V, Pagnoux C, Allanore Y, Launay D, Hachulla E, Authier FJ, Gherardi R, Kahan A, Cabane J, Guillevin L, Mouthon L. Myopathies related to systemic sclerosis: a case–control study of associated clinical and immunological features. Scand J Rheumatol. 2010;39(6):498–505. doi: 10.3109/03009741003774626. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

