Adaptation to prolonged neuromodulation in cortical cultures: an invariable return to network synchrony
- PMID: 25339462
- PMCID: PMC4237737
- DOI: 10.1186/s12915-014-0083-3
Adaptation to prolonged neuromodulation in cortical cultures: an invariable return to network synchrony
Abstract
Background: Prolonged neuromodulatory regimes, such as those critically involved in promoting arousal and suppressing sleep-associated synchronous activity patterns, might be expected to trigger adaptation processes and, consequently, a decline in neuromodulator-driven effects. This possibility, however, has rarely been addressed.
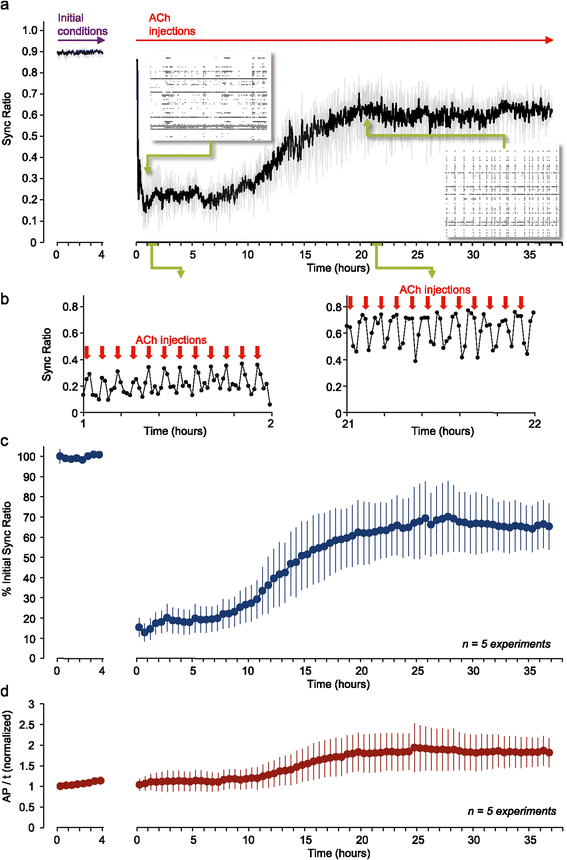
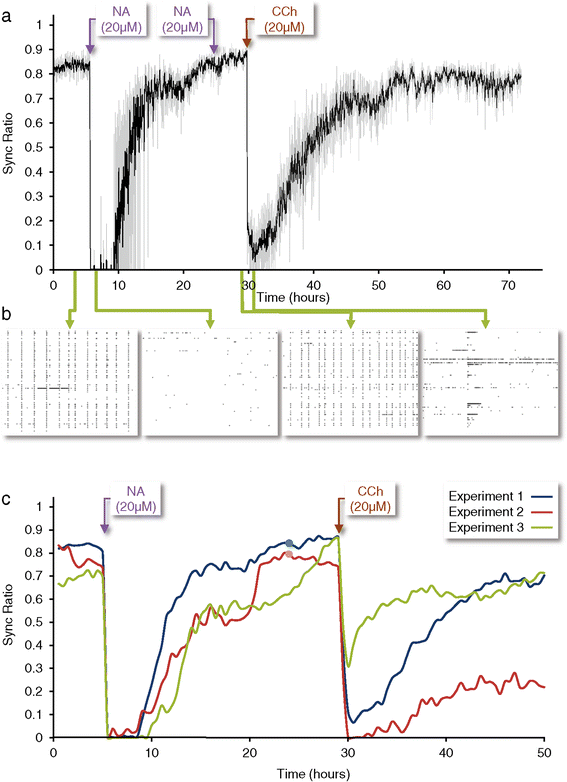
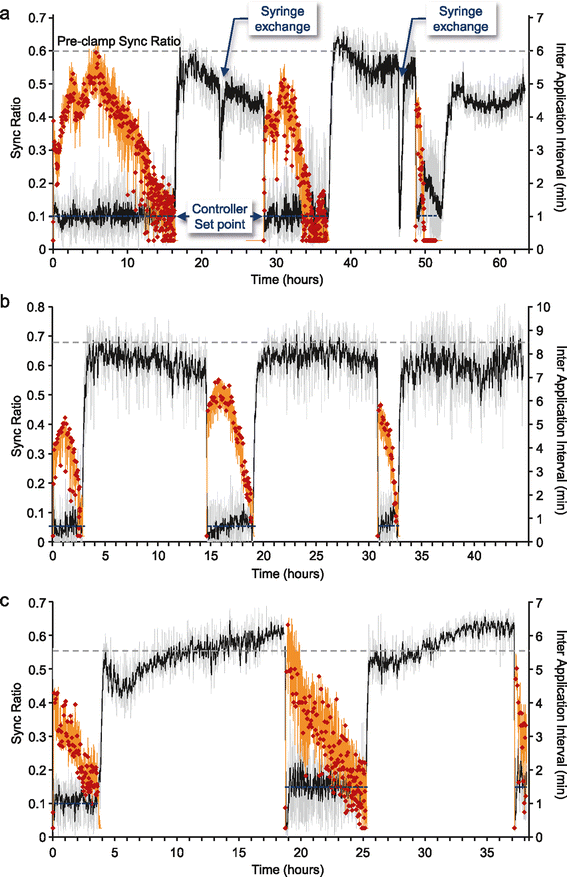
Results: Using networks of cultured cortical neurons, acetylcholine microinjections and a novel closed-loop 'synchrony-clamp' system, we found that acetylcholine pulses strongly suppressed network synchrony. Over the course of many hours, however, synchrony invariably reemerged, even when feedback was used to compensate for declining cholinergic efficacy. Network synchrony also reemerged following its initial suppression by noradrenaline, but this did not occlude the suppression of synchrony or its gradual reemergence following subsequent cholinergic input. Importantly, cholinergic efficacy could be restored and preserved over extended time scales by periodically withdrawing cholinergic input.
Conclusions: These findings indicate that the capacity of neuromodulators to suppress network synchrony is constrained by slow-acting, reactive processes. A multiplicity of neuromodulators and ultimately neuromodulator withdrawal periods might thus be necessary to cope with an inevitable reemergence of network synchrony.
Figures



Similar articles
-
Long-term relationships between cholinergic tone, synchronous bursting and synaptic remodeling.PLoS One. 2012;7(7):e40980. doi: 10.1371/journal.pone.0040980. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22911726 Free PMC article.
-
Nerve growth factor rapidly induces prolonged acetylcholine release from cultured basal forebrain neurons: differentiation between neuromodulatory and neurotrophic influences.J Neurosci. 2001 May 15;21(10):3375-82. doi: 10.1523/JNEUROSCI.21-10-03375.2001. J Neurosci. 2001. PMID: 11331367 Free PMC article.
-
Why Does Sleep Slow-Wave Activity Increase After Extended Wake? Assessing the Effects of Increased Cortical Firing During Wake and Sleep.J Neurosci. 2016 Dec 7;36(49):12436-12447. doi: 10.1523/JNEUROSCI.1614-16.2016. J Neurosci. 2016. PMID: 27927960 Free PMC article.
-
Neuromodulation and cortical function: modeling the physiological basis of behavior.Behav Brain Res. 1995 Feb;67(1):1-27. doi: 10.1016/0166-4328(94)00113-t. Behav Brain Res. 1995. PMID: 7748496 Review.
-
Directing neural plasticity to understand and treat tinnitus.Hear Res. 2013 Jan;295:58-66. doi: 10.1016/j.heares.2012.10.001. Epub 2012 Oct 23. Hear Res. 2013. PMID: 23099209 Review.
Cited by
-
Neuronal circuits overcome imbalance in excitation and inhibition by adjusting connection numbers.Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2018459118. doi: 10.1073/pnas.2018459118. Proc Natl Acad Sci U S A. 2021. PMID: 33723048 Free PMC article.
-
Coordinated Regulation of CB1 Cannabinoid Receptors and Anandamide Metabolism Stabilizes Network Activity during Homeostatic Downscaling.eNeuro. 2022 Nov 11;9(6):ENEURO.0276-22.2022. doi: 10.1523/ENEURO.0276-22.2022. Print 2022 Nov-Dec. eNeuro. 2022. PMID: 36316118 Free PMC article.
-
Canalization of genetic and pharmacological perturbations in developing primary neuronal activity patterns.Neuropharmacology. 2016 Jan;100:47-55. doi: 10.1016/j.neuropharm.2015.07.027. Epub 2015 Jul 26. Neuropharmacology. 2016. PMID: 26211975 Free PMC article.
-
A biophysical perspective on the resilience of neuronal excitability across timescales.Nat Rev Neurosci. 2023 Oct;24(10):640-652. doi: 10.1038/s41583-023-00730-9. Epub 2023 Aug 24. Nat Rev Neurosci. 2023. PMID: 37620600 Review.
-
Spontaneous and Perturbational Complexity in Cortical Cultures.Brain Sci. 2021 Nov 1;11(11):1453. doi: 10.3390/brainsci11111453. Brain Sci. 2021. PMID: 34827452 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

