Pro-B-type natriuretic peptide-1-108 processing and degradation in human heart failure
- PMID: 25339504
- PMCID: PMC4303547
- DOI: 10.1161/CIRCHEARTFAILURE.114.001174
Pro-B-type natriuretic peptide-1-108 processing and degradation in human heart failure
Abstract
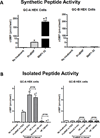
Background: We have reported that pro-B-type natriuretic peptide (BNP)-1-108 circulates and is processed to mature BNP1-32 in human blood. Building on these findings, we sought to determine whether proBNP1-108 processed forms in normal circulation are biologically active and stimulate cGMP, and whether proBNP1-108 processing and activity are altered in human heart failure (HF) compared with normal. Because BNP1-32 is deficient whereas proBNP1-108 is abundant in HF, we hypothesize that proBNP1-108 processing and degradation are impaired in HF patients ex vivo.
Methods and results: We measured circulating molecular forms, including BNP1-32, proBNP1-108, and N-terminal-proBNP, and all were significantly higher in patients with HF when compared with that in normals. Fresh serum samples from normals or patients with HF were incubated with or without exogenous nonglycosylated proBNP1-108 tagged with 6 C-terminal Histidines to facilitate peptide isolation. His-tag proBNP1-108 was efficiently processed into BNP1-32/3-32 at 5 minutes in normal serum, persisted for 15 minutes, then disappeared. Delayed processing of proBNP1-108 was observed in HF samples, and the degradation pattern differed depending on left ventricular function. The 5-minute processed forms from both normal and HF serums were active and generated cGMP via guanylyl cyclase-A receptors; however, the 180-minute samples were not active. The proBNP1-108 processing enzyme corin and BNP-degrading enzyme dipeptidyl peptidase-4 were reduced in HF versus normal, perhaps contributing to differential BNP metabolism in HF.
Conclusions: Exogenous proBNP1-108 is processed into active BNP1-32 and ultimately degraded in normal circulation. The processing and degradation of BNP molecular forms were altered but complete in HF, which may contribute to the pathophysiology of HF.
Keywords: B-type natriuretic peptide; enzymes; heart failure.
© 2014 American Heart Association, Inc.
Figures



Similar articles
-
BNP molecular forms and processing by the cardiac serine protease corin.Adv Clin Chem. 2013;61:1-31. doi: 10.1016/b978-0-12-407680-8.00001-4. Adv Clin Chem. 2013. PMID: 24015598 Free PMC article. Review.
-
Secretion of glycosylated pro-B-type natriuretic peptide from normal cardiomyocytes.Clin Chem. 2011 Jun;57(6):864-73. doi: 10.1373/clinchem.2010.157438. Epub 2011 Apr 11. Clin Chem. 2011. PMID: 21482747 Free PMC article.
-
Atrial and brain natriuretic peptides: Hormones secreted from the heart.Peptides. 2019 Jan;111:18-25. doi: 10.1016/j.peptides.2018.05.012. Epub 2018 May 31. Peptides. 2019. PMID: 29859763 Review.
-
Circulating forms of the b-type natriuretic peptide prohormone: pathophysiologic and clinical considerations.Adv Clin Chem. 2012;58:31-44. doi: 10.1016/b978-0-12-394383-5.00008-4. Adv Clin Chem. 2012. PMID: 22950341 Review.
-
Depletion of proBNP1-108 in patients with heart failure prevents cross-reactivity with natriuretic peptides.PLoS One. 2013 Sep 17;8(9):e75174. doi: 10.1371/journal.pone.0075174. eCollection 2013. PLoS One. 2013. PMID: 24069392 Free PMC article.
Cited by
-
Analytical Issues with Natriuretic Peptides - has this been Overly Simplified?EJIFCC. 2016 Aug 1;27(3):189-207. eCollection 2016 Aug. EJIFCC. 2016. PMID: 27683533 Free PMC article.
-
The Endocrine Function of the Heart: Physiology and Involvements of Natriuretic Peptides and Cyclic Nucleotide Phosphodiesterases in Heart Failure.J Clin Med. 2019 Oct 21;8(10):1746. doi: 10.3390/jcm8101746. J Clin Med. 2019. PMID: 31640161 Free PMC article. Review.
-
cGMP Signaling and Modulation in Heart Failure.J Cardiovasc Pharmacol. 2020 May;75(5):385-398. doi: 10.1097/FJC.0000000000000749. J Cardiovasc Pharmacol. 2020. PMID: 31464774 Free PMC article. Review.
-
Natriuretic peptide pathways in heart failure: further therapeutic possibilities.Cardiovasc Res. 2023 Feb 3;118(18):3416-3433. doi: 10.1093/cvr/cvac125. Cardiovasc Res. 2023. PMID: 36004816 Free PMC article.
-
Cyclic GMP modulating drugs in cardiovascular diseases: mechanism-based network pharmacology.Cardiovasc Res. 2022 Jul 20;118(9):2085-2102. doi: 10.1093/cvr/cvab240. Cardiovasc Res. 2022. PMID: 34270705 Free PMC article. Review.
References
-
- van den Akker F. Structural insights into the ligand binding domains of membrane bound guanylyl cyclases and natriuretic peptide receptors. J Mol Biol. 2001;311:923–937. - PubMed
-
- Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. - PubMed
-
- Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, Koshkina EV, Krasnoselsky MI, Katrukha AG. Processing of pro-b-type natriuretic peptide: Furin and corin as candidate convertases. Clin Chem. 2010;56:1166–1176. - PubMed
-
- Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, De Meester I. Dipeptidyl-peptidase iv converts intact b-type natriuretic peptide into its des-serpro form. Clin Chem. 2006;52:82–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

