Prolonged remission from hepatic encephalopathy with rifaximin: results of a placebo crossover analysis
- PMID: 25339518
- PMCID: PMC4284039
- DOI: 10.1111/apt.12993
Prolonged remission from hepatic encephalopathy with rifaximin: results of a placebo crossover analysis
Abstract
Background: Rifaximin therapy reduced risk of hepatic encephalopathy (HE) recurrence and HE-related hospitalisations during a 6-month, randomised, placebo-controlled trial (RCT) and a 24-month open-label maintenance (OLM) study. However, the impact of crossover from placebo to rifaximin therapy is unclear.
Aim: To study the impact of crossing over from placebo to rifaximin treatment on breakthrough HE and hospitalisation rates using a within-subjects design.
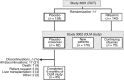
Methods: Adults with cirrhosis and history of overt HE episodes, currently in HE remission, received placebo during the RCT and crossed over to rifaximin 550 mg twice daily during the OLM study. Rate of breakthrough overt HE episodes, hospitalisations and incidence and rate of adverse events (AEs) were analysed during RCT and first 6 months of OLM.
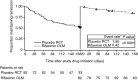
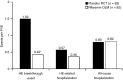
Results: Of 82 patients randomised to placebo in the RCT who crossed over to the OLM study, 39 experienced an HE episode during the RCT compared with 14 during the OLM study (P < 0.0001). Significantly lower rates of HE events were observed with rifaximin treatment compared with placebo treatment (P < 0.0001). Rates of HE-related hospitalisation were numerically lower during rifaximin treatment compared with placebo treatment, although not significant. Rates of most common AEs, serious AEs and infection-related AEs were similar between the two treatments.
Conclusions: This analysis confirms the repeatability of results from the RCT on safety and efficacy of rifaximin 550 mg twice daily in reducing the risk of hepatic encephalopathy recurrence, and suggests these findings are translatable outside of a rigorous, controlled trial setting.
© 2014 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
Comment in
-
Editorial: prolonged remission from hepatic encephalopathy with rifaximin.Aliment Pharmacol Ther. 2015 Jan;41(2):228. doi: 10.1111/apt.13025. Aliment Pharmacol Ther. 2015. PMID: 25511766 No abstract available.
References
-
- Ferenci P, Lockwood A, Mullen K. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. - PubMed
-
- Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034–41. - PubMed
-
- Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci. 2003;48:1622–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

