ABCG1 is required for pulmonary B-1 B cell and natural antibody homeostasis
- PMID: 25339664
- PMCID: PMC4239162
- DOI: 10.4049/jimmunol.1400606
ABCG1 is required for pulmonary B-1 B cell and natural antibody homeostasis
Abstract
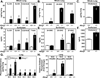
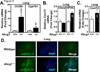
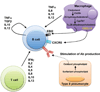
Many metabolic diseases, including atherosclerosis, type 2 diabetes, pulmonary alveolar proteinosis, and obesity, have a chronic inflammatory component involving both innate and adaptive immunity. Mice lacking the ATP-binding cassette transporter G1 (ABCG1) develop chronic inflammation in the lungs, which is associated with the lipid accumulation (cholesterol, cholesterol ester, and phospholipid) and cholesterol crystal deposition that are characteristic of atherosclerotic lesions and pulmonary alveolar proteinosis. In this article, we demonstrate that specific lipids, likely oxidized phospholipids and/or sterols, elicit a lung-specific immune response in Abcg1(-/-) mice. Loss of ABCG1 results in increased levels of specific oxysterols, phosphatidylcholines, and oxidized phospholipids, including 1-palmitoyl-2-(5'-oxovaleroyl)-sn-glycero-3-phosphocholine, in the lungs. Further, we identify a niche-specific increase in natural Ab (NAb)-secreting B-1 B cells in response to this lipid accumulation that is paralleled by increased titers of IgM, IgA, and IgG against oxidation-specific epitopes, such as those on oxidized low-density lipoprotein and malondialdehyde-modified low-density lipoprotein. Finally, we identify a cytokine/chemokine signature that is reflective of increased B cell activation, Ab secretion, and homing. Collectively, these data demonstrate that the accumulation of lipids in Abcg1(-/-) mice induces the specific expansion and localization of B-1 B cells, which secrete NAbs that may help to protect against the development of atherosclerosis. Indeed, despite chronic lipid accumulation and inflammation, hyperlipidemic mice lacking ABCG1 develop smaller atherosclerotic lesions compared with controls. These data also suggest that Abcg1(-/-) mice may represent a new model in which to study the protective functions of B-1 B cells/NAbs and suggest novel targets for pharmacologic intervention and treatment of disease.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no conflicts of interest or disclosures.
J.L.W. and X.Q. have patents and disclosures related to the use of oxidation-specific antibodies, which are owned by the University of California San Diego.
Figures






References
-
- Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. - PubMed
-
- Hardy RR. B-1 B Cell Development. J. Immunol. 2006;177:2749–2754. - PubMed
-
- Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol. Rev. 2000;175:70–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL088093/HL/NHLBI NIH HHS/United States
- R21 HL111906/HL/NHLBI NIH HHS/United States
- HL088093/HL/NHLBI NIH HHS/United States
- U54 GM069338/GM/NIGMS NIH HHS/United States
- HL074214/HL/NHLBI NIH HHS/United States
- K99 HL118161/HL/NHLBI NIH HHS/United States
- R01 HL107794/HL/NHLBI NIH HHS/United States
- R01 HL074214/HL/NHLBI NIH HHS/United States
- HL107794/HL/NHLBI NIH HHS/United States
- GM69338-06/GM/NIGMS NIH HHS/United States
- R00 HL118161/HL/NHLBI NIH HHS/United States
- HL111906/HL/NHLBI NIH HHS/United States
- P01 HL030568/HL/NHLBI NIH HHS/United States
- HL118161/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

