DOCK2 and DOCK5 act additively in neutrophils to regulate chemotaxis, superoxide production, and extracellular trap formation
- PMID: 25339677
- PMCID: PMC4640904
- DOI: 10.4049/jimmunol.1400885
DOCK2 and DOCK5 act additively in neutrophils to regulate chemotaxis, superoxide production, and extracellular trap formation
Abstract
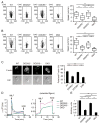
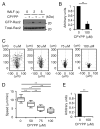
Neutrophils are highly motile leukocytes that play important roles in the innate immune response to invading pathogens. Neutrophils rapidly migrate to the site of infections and kill pathogens by producing reactive oxygen species (ROS). Neutrophil chemotaxis and ROS production require activation of Rac small GTPase. DOCK2, an atypical guanine nucleotide exchange factor (GEF), is one of the major regulators of Rac in neutrophils. However, because DOCK2 deficiency does not completely abolish fMLF-induced Rac activation, other Rac GEFs may also participate in this process. In this study, we show that DOCK5 acts with DOCK2 in neutrophils to regulate multiple cellular functions. We found that fMLF- and PMA-induced Rac activation were almost completely lost in mouse neutrophils lacking both DOCK2 and DOCK5. Although β2 integrin-mediated adhesion occurred normally even in the absence of DOCK2 and DOCK5, mouse neutrophils lacking DOCK2 and DOCK5 exhibited a severe defect in chemotaxis and ROS production. Similar results were obtained when human neutrophils were treated with CPYPP, a small-molecule inhibitor of these DOCK GEFs. Additionally, we found that DOCK2 and DOCK5 regulate formation of neutrophil extracellular traps (NETs). Because NETs are involved in vascular inflammation and autoimmune responses, DOCK2 and DOCK5 would be a therapeutic target for controlling NET-mediated inflammatory disorders.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



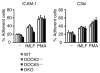
References
-
- Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. - PubMed
-
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. - PubMed
-
- Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. - PubMed
-
- Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, Levine JE, Petryniak B, Derrow CW, Harris C, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. - PubMed
-
- Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169:5043–5051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

