Calcium spike-mediated digital signaling increases glutamate output at the visual threshold of retinal bipolar cells
- PMID: 25339710
- PMCID: PMC4297791
- DOI: 10.1152/jn.00378.2014
Calcium spike-mediated digital signaling increases glutamate output at the visual threshold of retinal bipolar cells
Abstract
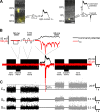
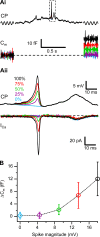
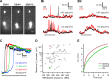
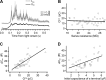
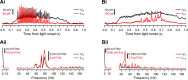
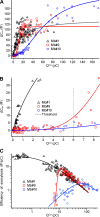
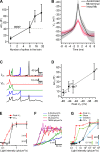
Most retinal bipolar cells (BCs) transmit visual input from photoreceptors to ganglion cells using graded potentials, but some also generate calcium or sodium spikes. Sodium spikes are thought to increase temporal precision of light-evoked BC signaling; however, the role of calcium spikes in BCs is not fully understood. Here we studied how calcium spikes and graded responses mediate neurotransmitter release from Mb-type BCs, known to produce both. In dark-adapted goldfish retinal slices, light induced spikes in 40% of the axon terminals of intact Mbs; in the rest, light generated graded responses. These light-evoked membrane potentials were used to depolarize axotomized Mb terminals where depolarization-evoked calcium current (ICa) and consequent exocytosis-associated membrane capacitance increases (ΔCm) could be precisely measured. When evoked by identical dim light intensities, spiking responses transferred more calcium (Q(Ca)) and triggered larger exocytosis with higher efficiency (ΔCm/Q(Ca)) than graded potentials. Q(Ca) was translated into exocytosis linearly when transferred with spikes and supralinearly when transferred with graded responses. At the Mb output (ΔCm), spiking responses coded light intensity with numbers and amplitude whereas graded responses coded with amplitude, duration, and steepness. Importantly, spiking responses saturated exocytosis within scotopic range but graded potentials did not. We propose that calcium spikes in Mbs increase signal input-output ratio by boosting Mb glutamate release at threshold intensities. Therefore, spiking Mb responses are suitable to transfer low-light-intensity signals to ganglion cells with higher gain, whereas graded potentials signal for light over a wider range of intensities at the Mb output.
Keywords: bipolar cell; calcium spike; graded potential; light response; threshold.
Copyright © 2015 the American Physiological Society.
Figures



Similar articles
-
Quantifying the effect of light activated outer and inner retinal inhibitory pathways on glutamate release from mixed bipolar cells.Synapse. 2018 May;72(5):e22028. doi: 10.1002/syn.22028. Epub 2018 Feb 2. Synapse. 2018. PMID: 29360185
-
Nitric oxide mediates activity-dependent plasticity of retinal bipolar cell output via S-nitrosylation.J Neurosci. 2013 Dec 4;33(49):19176-93. doi: 10.1523/JNEUROSCI.2792-13.2013. J Neurosci. 2013. PMID: 24305814 Free PMC article.
-
Spikes in retinal bipolar cells phase-lock to visual stimuli with millisecond precision.Curr Biol. 2011 Nov 22;21(22):1859-69. doi: 10.1016/j.cub.2011.09.042. Epub 2011 Nov 3. Curr Biol. 2011. PMID: 22055291 Free PMC article.
-
Synaptic release at mammalian bipolar cell terminals.Vis Neurosci. 2011 Jan;28(1):109-19. doi: 10.1017/S0952523810000453. Vis Neurosci. 2011. PMID: 21272392 Free PMC article. Review.
-
Spikes and ribbon synapses in early vision.Trends Neurosci. 2013 Aug;36(8):480-8. doi: 10.1016/j.tins.2013.04.006. Epub 2013 May 22. Trends Neurosci. 2013. PMID: 23706152 Review.
Cited by
-
Postsynaptic Plasticity Triggered by Ca²⁺-Permeable AMPA Receptor Activation in Retinal Amacrine Cells.Neuron. 2016 Feb 3;89(3):507-20. doi: 10.1016/j.neuron.2015.12.028. Epub 2016 Jan 21. Neuron. 2016. PMID: 26804991 Free PMC article.
-
Calcium- and Voltage-Dependent Dual Gating ANO1 is an Intrinsic Determinant of Repolarization in Rod Bipolar Cells of the Mouse Retina.Cells. 2020 Feb 26;9(3):543. doi: 10.3390/cells9030543. Cells. 2020. PMID: 32110998 Free PMC article.
-
Inhibitory input to the direction-selective ganglion cell is saturated at low contrast.J Neurophysiol. 2015 Aug;114(2):927-41. doi: 10.1152/jn.00413.2015. Epub 2015 Jun 10. J Neurophysiol. 2015. PMID: 26063782 Free PMC article.
-
Graded spikes differentially signal neurotransmitter input in cerebrospinal fluid contacting neurons of the mouse spinal cord.iScience. 2022 Dec 30;26(1):105914. doi: 10.1016/j.isci.2022.105914. eCollection 2023 Jan 20. iScience. 2022. PMID: 36691620 Free PMC article.
References
-
- Baden T, Berens P, Bethge M, Euler T. Spikes in mammalian bipolar cells support temporal layering of the inner retina. Curr Biol 23: 48–52, 2013a. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

