Effects of HIV-1 Tat on enteric neuropathogenesis
- PMID: 25339738
- PMCID: PMC4205550
- DOI: 10.1523/JNEUROSCI.2283-14.2014
Effects of HIV-1 Tat on enteric neuropathogenesis
Abstract
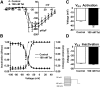
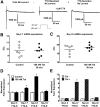
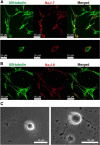
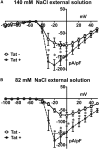
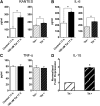
The gastrointestinal (GI) tract presents a major site of immune modulation by HIV, resulting in significant morbidity. Most GI processes affected during HIV infection are regulated by the enteric nervous system. HIV has been identified in GI histologic specimens in up to 40% of patients, and the presence of viral proteins, including the trans-activator of transcription (Tat), has been reported in the gut indicating that HIV itself may be an indirect gut pathogen. Little is known of how Tat affects the enteric nervous system. Here we investigated the effects of the Tat protein on enteric neuronal excitability, proinflammatory cytokine release, and its overall effect on GI motility. Direct application of Tat (100 nm) increased the number of action potentials and reduced the threshold for action potential initiation in isolated myenteric neurons. This effect persisted in neurons pretreated with Tat for 3 d (19 of 20) and in neurons isolated from Tat(+) (Tat-expressing) transgenic mice. Tat increased sodium channel isoforms Nav1.7 and Nav1.8 levels. This increase was accompanied by an increase in sodium current density and a leftward shift in the sodium channel activation voltage. RANTES, IL-6, and IL-1β, but not TNF-α, were enhanced by Tat. Intestinal transit and cecal water content were also significantly higher in Tat(+) transgenic mice than Tat(-) littermates (controls). Together, these findings show that Tat has a direct and persistent effect on enteric neuronal excitability, and together with its effect on proinflammatory cytokines, regulates gut motility, thereby contributing to GI dysmotilities reported in HIV patients.
Keywords: AIDS; HIV; cytokines; myenteric; sodium channels.
Copyright © 2014 the authors 0270-6474/14/3414243-09$15.00/0.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
