PGC-1α provides a transcriptional framework for synchronous neurotransmitter release from parvalbumin-positive interneurons
- PMID: 25339750
- PMCID: PMC4205559
- DOI: 10.1523/JNEUROSCI.1222-14.2014
PGC-1α provides a transcriptional framework for synchronous neurotransmitter release from parvalbumin-positive interneurons
Abstract
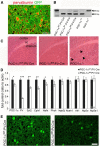
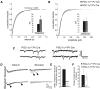
Accumulating evidence strongly implicates the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) in the pathophysiology of multiple neurological disorders, but the downstream gene targets of PGC-1α in the brain have remained enigmatic. Previous data demonstrate that PGC-1α is primarily concentrated in inhibitory neurons and that PGC-1α is required for the expression of the interneuron-specific Ca(2+)-binding protein parvalbumin (PV) throughout the cortex. To identify other possible transcriptional targets of PGC-1α in neural tissue, we conducted a microarray on neuroblastoma cells overexpressing PGC-1α, mined results for genes with physiological relevance to interneurons, and measured cortical gene and protein expression of these genes in mice with underexpression and overexpression of PGC-1α. We observed bidirectional regulation of novel PGC-1α-dependent transcripts spanning synaptic [synaptotagmin 2 (Syt2) and complexin 1 (Cplx1)], structural [neurofilament heavy chain (Nefh)], and metabolic [neutral cholesterol ester hydrolase 1 (Nceh1), adenylate kinase 1 (Ak1), inositol polyphosphate 5-phosphatase J (Inpp5j), ATP synthase mitochondrial F1 complex O subunit (Atp5o), phytanol-CoA-2hydroxylase (Phyh), and ATP synthase mitrochondrial F1 complex α subunit 1 (Atp5a1)] functions. The neuron-specific genes Syt2, Cplx1, and Nefh were developmentally upregulated in an expression pattern consistent with that of PGC-1α and were expressed in cortical interneurons. Conditional deletion of PGC-1α in PV-positive neurons significantly decreased cortical transcript expression of these genes, promoted asynchronous GABA release, and impaired long-term memory. Collectively, these data demonstrate that PGC-1α is required for normal PV-positive interneuron function and that loss of PGC-1α in this interneuron subpopulation could contribute to cortical dysfunction in disease states.
Keywords: Barnes maze; Huntington disease; cortical development; ppargc1a; schizophrenia; strontium.
Copyright © 2014 the authors 0270-6474/14/3414375-13$15.00/0.
Figures






References
-
- Alcántara S, Ferrer I, Soriano E. Postnatal development of parvalbumin and calbindin D28K immunoreactivities in the cerebral cortex of the rat. Anat Embryol. 1993;188:63–73. - PubMed
-
- Behrends JC, ten Bruggencate G. Changes in quantal size distributions upon experimental variations in the probability of release at striatal inhibitory synapses. J Neurophysiol. 1998;79:2999–3011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
