The sheep tetherin paralog oBST2B blocks envelope glycoprotein incorporation into nascent retroviral virions
- PMID: 25339764
- PMCID: PMC4301133
- DOI: 10.1128/JVI.02751-14
The sheep tetherin paralog oBST2B blocks envelope glycoprotein incorporation into nascent retroviral virions
Abstract
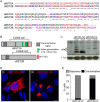
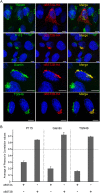
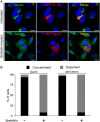
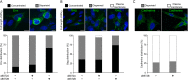
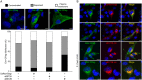
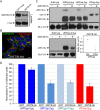
Bone marrow stromal cell antigen 2 (BST2) is a cellular restriction factor with a broad antiviral activity. In sheep, the BST2 gene is duplicated into two paralogs termed oBST2A and oBST2B. oBST2A impedes viral exit of the Jaagsiekte sheep retroviruses (JSRV), most probably by retaining virions at the cell membrane, similar to the "tethering" mechanism exerted by human BST2. In this study, we provide evidence that unlike oBST2A, oBST2B is limited to the Golgi apparatus and disrupts JSRV envelope (Env) trafficking by sequestering it. In turn, oBST2B leads to a reduction in Env incorporation into viral particles, which ultimately results in the release of virions that are less infectious. Furthermore, the activity of oBST2B does not seem to be restricted to retroviruses, as it also acts on vesicular stomatitis virus glycoproteins. Therefore, we suggest that oBST2B exerts antiviral activity using a mechanism distinct from the classical tethering restriction observed for oBST2A.
Importance: BST2 is a powerful cellular restriction factor against a wide range of enveloped viruses. Sheep possess two paralogs of the BST2 gene called oBST2A and oBST2B. JSRV, the causative agent of a transmissible lung cancer of sheep, is known to be restricted by oBST2A. In this study, we show that unlike oBST2A, oBST2B impairs the normal cellular trafficking of JSRV envelope glycoproteins by sequestering them within the Golgi apparatus. We also show that oBST2B decreases the incorporation of envelope glycoprotein into JSRV viral particles, which in turn reduces virion infectivity. In conclusion, oBST2B exerts a novel antiviral activity that is distinct from those of BST2 proteins of other species.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. doi: 10.1016/j.chom.2008.03.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

