CLC channel function and dysfunction in health and disease
- PMID: 25339907
- PMCID: PMC4188032
- DOI: 10.3389/fphys.2014.00378
CLC channel function and dysfunction in health and disease
Abstract
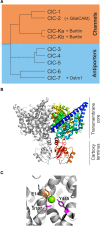
CLC channels and transporters are expressed in most tissues and fulfill diverse functions. There are four human CLC channels, ClC-1, ClC-2, ClC-Ka, and ClC-Kb, and five CLC transporters, ClC-3 through -7. Some of the CLC channels additionally associate with accessory subunits. Whereas barttin is mandatory for the functional expression of ClC-K, GlialCam is a facultative subunit of ClC-2 which modifies gating and thus increases the functional variability within the CLC family. Isoform-specific ion conduction and gating properties optimize distinct CLC channels for their cellular tasks. ClC-1 preferentially conducts at negative voltages, and the resulting inward rectification provides a large resting chloride conductance without interference with the muscle action potential. Exclusive opening at voltages negative to the chloride reversal potential allows for ClC-2 to regulate intracellular chloride concentrations. ClC-Ka and ClC-Kb are equally suited for inward and outward currents to support transcellular chloride fluxes. Every human CLC channel gene has been linked to a genetic disease, and studying these mutations has provided much information about the physiological roles and the molecular basis of CLC channel function. Mutations in the gene encoding ClC-1 cause myotonia congenita, a disease characterized by sarcolemmal hyperexcitability and muscle stiffness. Loss-of-function of ClC-Kb/barttin channels impairs NaCl resorption in the limb of Henle and causes hyponatriaemia, hypovolemia and hypotension in patients suffering from Bartter syndrome. Mutations in CLCN2 were found in patients with CNS disorders but the functional role of this isoform is still not understood. Recent links between ClC-1 and epilepsy and ClC-Ka and heart failure suggested novel cellular functions of these proteins. This review aims to survey the knowledge about physiological and pathophysiological functions of human CLC channels in the light of recent discoveries from biophysical, physiological, and genetic studies.
Keywords: Bartter syndrome; CLC channel; anion channel; leukencephalopathy; myotonia congenita; patch clamp.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

