Skeletal muscle pathology in Huntington's disease
- PMID: 25339908
- PMCID: PMC4186279
- DOI: 10.3389/fphys.2014.00380
Skeletal muscle pathology in Huntington's disease
Abstract
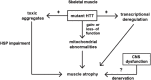
Huntington's disease (HD) is a hereditary neurodegenerative disorder caused by the expansion of a polyglutamine stretch within the huntingtin protein (HTT). The neurological symptoms, that involve motor, cognitive and psychiatric disturbances, are caused by neurodegeneration that is particularly widespread in the basal ganglia and cereberal cortex. HTT is ubiquitously expressed and in recent years it has become apparent that HD patients experience a wide array of peripheral organ dysfunction including severe metabolic phenotype, weight loss, HD-related cardiomyopathy and skeletal muscle wasting. Although skeletal muscles pathology became a hallmark of HD, the mechanisms underlying muscular atrophy in this disorder are unknown. Skeletal muscles account for approximately 40% of body mass and are highly adaptive to physiological and pathological conditions that may result in muscle hypertrophy (due to increased mechanical load) or atrophy (inactivity, chronic disease states). The atrophy is caused by degeneration of myofibers and their replacement by fibrotic tissue is the major pathological feature in many genetic muscle disorders. Under normal physiological conditions the muscle function is orchestrated by a network of intrinsic hypertrophic and atrophic signals linked to the functional properties of the motor units that are likely to be imbalanced in HD. In this article, we highlight the emerging field of research with particular focus on the recent studies of the skeletal muscle pathology and the identification of new disease-modifying treatments.
Keywords: Huntington's disease; disease modifying treatment; peripheral pathology; skeletal muscle atrophy.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources