Evolution of B cell immunity
- PMID: 25340015
- PMCID: PMC4203447
- DOI: 10.1146/annurev-animal-031412-103651
Evolution of B cell immunity
Abstract
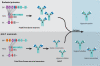
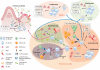
Two types of adaptive immune strategies are known to have evolved in vertebrates: the VLR-based system, which is present in jawless organisms and is mediated by VLRA and VLRB lymphocytes, and the BCR/TCR-based system, which is present in jawed species and is provided by B and T cell receptors expressed on B and T cells, respectively. Here we summarize features of B cells and their predecessors in the different animal phyla, focusing the review on B cells from jawed vertebrates. We point out the critical role of nonclassical species and comparative immunology studies in the understanding of B cell immunity. Because nonclassical models include species relevant to veterinary medicine, basic science research performed in these animals contributes to the knowledge required for the development of more efficacious vaccines against emerging pathogens.
Figures

Similar articles
-
Genomic donor cassette sharing during VLRA and VLRC assembly in jawless vertebrates.Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14828-33. doi: 10.1073/pnas.1415580111. Epub 2014 Sep 16. Proc Natl Acad Sci U S A. 2014. PMID: 25228758 Free PMC article.
-
Immunological Diversity Is a Cornerstone of Organismal Defense and Allorecognition across Metazoa.J Immunol. 2022 Jan 15;208(2):203-211. doi: 10.4049/jimmunol.2100754. J Immunol. 2022. PMID: 35017209 Review.
-
Comparative immune systems in animals.Annu Rev Anim Biosci. 2014 Feb;2:235-58. doi: 10.1146/annurev-animal-031412-103634. Epub 2013 Nov 14. Annu Rev Anim Biosci. 2014. PMID: 25384142 Review.
-
Evolution of innate and adaptive immune systems in jawless vertebrates.Microbiol Immunol. 2013 Jan;57(1):1-12. doi: 10.1111/j.1348-0421.2012.00500.x. Microbiol Immunol. 2013. PMID: 22924515 Review.
-
The evolution of adaptive immunity in vertebrates.Adv Immunol. 2011;109:125-57. doi: 10.1016/B978-0-12-387664-5.00004-2. Adv Immunol. 2011. PMID: 21569914 Review.
Cited by
-
The immunoregulation of mesenchymal stem cells plays a critical role in improving the prognosis of liver transplantation.J Transl Med. 2019 Dec 10;17(1):412. doi: 10.1186/s12967-019-02167-0. J Transl Med. 2019. PMID: 31823784 Free PMC article. Review.
-
The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies.Front Immunol. 2021 Nov 19;12:791081. doi: 10.3389/fimmu.2021.791081. eCollection 2021. Front Immunol. 2021. PMID: 34868080 Free PMC article. Review.
-
Regulation of B cell fate, survival, and function by mitochondria and autophagy.Mitochondrion. 2018 Jul;41:58-65. doi: 10.1016/j.mito.2017.11.005. Epub 2017 Nov 23. Mitochondrion. 2018. PMID: 29175010 Free PMC article. Review.
-
Destabilisation of T cell-dependent humoral immunity in sepsis.Clin Sci (Lond). 2024 Jan 10;138(1):65-85. doi: 10.1042/CS20230517. Clin Sci (Lond). 2024. PMID: 38197178 Free PMC article. Review.
-
RNA-Seq of Single Fish Cells - Seeking Out the Leukocytes Mediating Immunity in Teleost Fishes.Front Immunol. 2022 Jan 24;13:798712. doi: 10.3389/fimmu.2022.798712. eCollection 2022. Front Immunol. 2022. PMID: 35140719 Free PMC article. Review.
References
-
- Bhaya D, Davison M, Barrangou R. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 2011;45:273–97. - PubMed
-
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. - PubMed
-
- Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu. Rev. Immunol. 2006;24:497–518. - PubMed
-
- Matsunaga T, Rahman A. What brought the adaptive immune system to vertebrates?—The jaw hypothesis and the seahorse. Immunol. Rev. 1998;166:177–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
