Parts & pools: a framework for modular design of synthetic gene circuits
- PMID: 25340051
- PMCID: PMC4186347
- DOI: 10.3389/fbioe.2014.00042
Parts & pools: a framework for modular design of synthetic gene circuits
Abstract
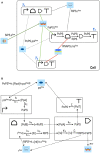
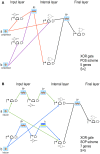
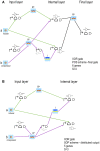
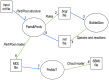
Published in 2008, Parts & Pools represents one of the first attempts to conceptualize the modular design of bacterial synthetic gene circuits with Standard Biological Parts (DNA segments) and Pools of molecules referred to as common signal carriers (e.g., RNA polymerases and ribosomes). The original framework for modeling bacterial components and designing prokaryotic circuits evolved over the last years and brought, first, to the development of an algorithm for the automatic design of Boolean gene circuits. This is a remarkable achievement since gene digital circuits have a broad range of applications that goes from biosensors for health and environment care to computational devices. More recently, Parts & Pools was enabled to give a proper formal description of eukaryotic biological circuit components. This was possible by employing a rule-based modeling approach, a technique that permits a faithful calculation of all the species and reactions involved in complex systems such as eukaryotic cells and compartments. In this way, Parts & Pools is currently suitable for the visual and modular design of synthetic gene circuits in yeast and mammalian cells too.
Keywords: Boolean gates; Parts; Pools; gene circuits; modeling; synthetic biology.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

