Keratin 76 is required for tight junction function and maintenance of the skin barrier
- PMID: 25340345
- PMCID: PMC4207637
- DOI: 10.1371/journal.pgen.1004706
Keratin 76 is required for tight junction function and maintenance of the skin barrier
Abstract
Keratins are cytoskeletal intermediate filament proteins that are increasingly being recognised for their diverse cellular functions. Here we report the consequences of germ line inactivation of Keratin 76 (Krt76) in mice. Homozygous disruption of this epidermally expressed gene causes neonatal skin flaking, hyperpigmentation, inflammation, impaired wound healing, and death prior to 12 weeks of age. We show that this phenotype is associated with functionally defective tight junctions that are characterised by mislocalization of the integral protein CLDN1. We further demonstrate that KRT76 interacts with CLDN1 and propose that this interaction is necessary to correctly position CLDN1 in tight junctions. The mislocalization of CLDN1 has been associated in various dermopathies, including the inflammatory disease, psoriasis. These observations establish a previously unknown connection between the intermediate filament cytoskeleton network and tight junctions and showcase Krt76 null mice as a possible model to study aberrant tight junction driven skin diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

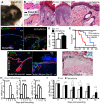

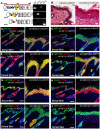
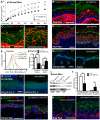
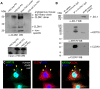
Similar articles
-
CLDN1 knock out keratinocytes as a model to investigate multiple skin disorders.Exp Dermatol. 2024 May;33(5):e15084. doi: 10.1111/exd.15084. Exp Dermatol. 2024. PMID: 38711223
-
Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice.J Dermatol Sci. 2013 Apr;70(1):12-8. doi: 10.1016/j.jdermsci.2013.01.002. Epub 2013 Jan 31. J Dermatol Sci. 2013. PMID: 23433550
-
Diseases of epidermal keratins and their linker proteins.Exp Cell Res. 2007 Jun 10;313(10):1995-2009. doi: 10.1016/j.yexcr.2007.03.029. Epub 2007 Apr 24. Exp Cell Res. 2007. PMID: 17531221 Free PMC article. Review.
-
Differential Expression and Function of Bicellular Tight Junctions in Skin and Oral Wound Healing.Int J Mol Sci. 2020 Apr 23;21(8):2966. doi: 10.3390/ijms21082966. Int J Mol Sci. 2020. PMID: 32340108 Free PMC article.
-
Keratins and skin disorders.Cell Biol Int. 1996 Apr;20(4):261-74. Cell Biol Int. 1996. PMID: 8664850 Review.
Cited by
-
Distinctive genes and signaling pathways associated with type 2 diabetes-related periodontitis: Preliminary study.PLoS One. 2024 Jan 19;19(1):e0296925. doi: 10.1371/journal.pone.0296925. eCollection 2024. PLoS One. 2024. PMID: 38241313 Free PMC article.
-
Topical Aminosalicylic Acid Improves Keratinocyte Differentiation in an Inducible Mouse Model of Harlequin Ichthyosis.Cell Rep Med. 2020 Nov 17;1(8):100129. doi: 10.1016/j.xcrm.2020.100129. eCollection 2020 Nov 17. Cell Rep Med. 2020. PMID: 33294854 Free PMC article.
-
Cell Adhesion at the Tight Junctions: New Aspects and New Functions.Cells. 2023 Nov 24;12(23):2701. doi: 10.3390/cells12232701. Cells. 2023. PMID: 38067129 Free PMC article. Review.
-
Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity.Tissue Barriers. 2016 May 2;4(3):e1178368. doi: 10.1080/21688370.2016.1178368. eCollection 2016 Jul-Sep. Tissue Barriers. 2016. PMID: 27583190 Free PMC article. Review.
-
Systems Proteomics View of the Endogenous Human Claudin Protein Family.J Proteome Res. 2016 Feb 5;15(2):339-59. doi: 10.1021/acs.jproteome.5b00769. Epub 2016 Jan 12. J Proteome Res. 2016. PMID: 26680015 Free PMC article. Review.
References
-
- Fuchs E, Weber K (1994) Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 63: 345–382. - PubMed
-
- Lane EB, McLean WH (2004) Keratins and skin disorders. J Pathol 204: 355–366. - PubMed
-
- Parry DA, Strelkov SV, Burkhard P, Aebi U, Herrmann H (2007) Towards a molecular description of intermediate filament structure and assembly. Exp Cell Res 313: 2204–2216. - PubMed
-
- Fuchs E, Cleveland DW (1998) A structural scaffolding of intermediate filaments in health and disease. Science 279: 514–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

