Molecular modeling of a tandem two pore domain potassium channel reveals a putative binding site for general anesthetics
- PMID: 25340635
- PMCID: PMC4306477
- DOI: 10.1021/cn500172e
Molecular modeling of a tandem two pore domain potassium channel reveals a putative binding site for general anesthetics
Abstract
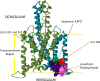
Anesthetics are thought to mediate a portion of their activity via binding to and modulation of potassium channels. In particular, tandem pore potassium channels (K2P) are transmembrane ion channels whose current is modulated by the presence of general anesthetics and whose genetic absence has been shown to confer a level of anesthetic resistance. While the exact molecular structure of all K2P forms remains unknown, significant progress has been made toward understanding their structure and interactions with anesthetics via the methods of molecular modeling, coupled with the recently released higher resolution structures of homologous potassium channels to act as templates. Such models reveal the convergence of amino acid regions that are known to modulate anesthetic activity onto a common three- dimensional cavity that forms a putative anesthetic binding site. The model successfully predicts additional important residues that are also involved in the putative binding site as validated by the results of suggested experimental mutations. Such a model can now be used to further predict other amino acid residues that may be intimately involved in the target-based structure-activity relationships that are necessary for anesthetic binding.
Keywords: Tandem pore potassium channel; anesthesia; homology modeling.
Figures



Similar articles
-
Determinants of the anesthetic sensitivity of two-pore domain acid-sensitive potassium channels: molecular cloning of an anesthetic-activated potassium channel from Lymnaea stagnalis.J Biol Chem. 2007 Jul 20;282(29):20977-90. doi: 10.1074/jbc.M610692200. Epub 2007 Jun 4. J Biol Chem. 2007. PMID: 17548360
-
Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action.J Biol Chem. 2002 May 17;277(20):17733-42. doi: 10.1074/jbc.M200502200. Epub 2002 Mar 8. J Biol Chem. 2002. PMID: 11886861
-
Molecular mapping of general anesthetic sites in a voltage-gated ion channel.Biophys J. 2011 Oct 5;101(7):1613-22. doi: 10.1016/j.bpj.2011.08.026. Biophys J. 2011. PMID: 21961587 Free PMC article.
-
The TREK K2P channels and their role in general anaesthesia and neuroprotection.Trends Pharmacol Sci. 2004 Nov;25(11):601-8. doi: 10.1016/j.tips.2004.09.003. Trends Pharmacol Sci. 2004. PMID: 15491783 Review.
-
Therapeutic potential of neuronal two-pore domain potassium-channel modulators.Curr Opin Investig Drugs. 2007 Jul;8(7):555-62. Curr Opin Investig Drugs. 2007. PMID: 17659475 Review.
Cited by
-
The CNS under pathophysiologic attack--examining the role of K₂p channels.Pflugers Arch. 2015 May;467(5):959-72. doi: 10.1007/s00424-014-1664-2. Epub 2014 Dec 9. Pflugers Arch. 2015. PMID: 25482672 Review.
-
Breathing Stimulant Compounds Inhibit TASK-3 Potassium Channel Function Likely by Binding at a Common Site in the Channel Pore.Mol Pharmacol. 2015 Nov;88(5):926-34. doi: 10.1124/mol.115.100107. Epub 2015 Aug 12. Mol Pharmacol. 2015. PMID: 26268529 Free PMC article.
-
Studies on the mechanism of general anesthesia.Proc Natl Acad Sci U S A. 2020 Jun 16;117(24):13757-13766. doi: 10.1073/pnas.2004259117. Epub 2020 May 28. Proc Natl Acad Sci U S A. 2020. PMID: 32467161 Free PMC article.
-
How to resolve microsecond current fluctuations in single ion channels: the power of beta distributions.Channels (Austin). 2015;9(5):262-80. doi: 10.1080/19336950.2015.1083660. Channels (Austin). 2015. PMID: 26368656 Free PMC article. Review.
-
Disruption of palmitate-mediated localization; a shared pathway of force and anesthetic activation of TREK-1 channels.Biochim Biophys Acta Biomembr. 2020 Jan 1;1862(1):183091. doi: 10.1016/j.bbamem.2019.183091. Epub 2019 Oct 28. Biochim Biophys Acta Biomembr. 2020. PMID: 31672538 Free PMC article. Review.
References
-
- Franks N. P.; Honore E. (2004) The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol. Sci. 25, 601–608. - PubMed
-
- Lesage F.; Lazdunski M. (2000) Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol.: Renal Physiol. 279, F793–801. - PubMed
-
- Linden A. M.; Aller M. I.; Leppa E.; Vekovischeva O.; Aitta-Aho T.; Veale E. L.; Mathie A.; Rosenberg P.; Wisden W.; Korpi E. R. (2006) The in vivo contributions of TASK-1-containing channels to the actions of inhalation anesthetics, the alpha(2) adrenergic sedative dexmedetomidine, and cannabinoid agonists. J. Pharmacol. Exp. Ther. 317, 615–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

