Genetic influences on translation in yeast
- PMID: 25340754
- PMCID: PMC4207643
- DOI: 10.1371/journal.pgen.1004692
Genetic influences on translation in yeast
Abstract
Heritable differences in gene expression between individuals are an important source of phenotypic variation. The question of how closely the effects of genetic variation on protein levels mirror those on mRNA levels remains open. Here, we addressed this question by using ribosome profiling to examine how genetic differences between two strains of the yeast S. cerevisiae affect translation. Strain differences in translation were observed for hundreds of genes. Allele specific measurements in the diploid hybrid between the two strains revealed roughly half as many cis-acting effects on translation as were observed for mRNA levels. In both the parents and the hybrid, most effects on translation were of small magnitude, such that the direction of an mRNA difference was typically reflected in a concordant footprint difference. The relative importance of cis and trans acting variation on footprint levels was similar to that for mRNA levels. There was a tendency for translation to cause larger footprint differences than expected given the respective mRNA differences. This is in contrast to translational differences between yeast species that have been reported to more often oppose than reinforce mRNA differences. Finally, we catalogued instances of premature translation termination in the two yeast strains and also found several instances where erroneous reference gene annotations lead to apparent nonsense mutations that in fact reside outside of the translated gene body. Overall, genetic influences on translation subtly modulate gene expression differences, and translation does not create strong discrepancies between genetic influences on mRNA and protein levels.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

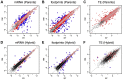
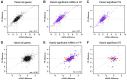
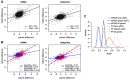
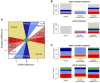
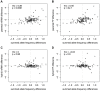
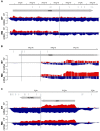
References
-
- Brem RB, Yvert G, Clinton R, Kruglyak L (2002) Genetic Dissection of Transcriptional Regulation in Budding Yeast. Science (New York, NY) 296: 752–755. - PubMed
-
- Rockman MV, Kruglyak L (2006) Genetics of global gene expression. Nature Reviews Genetics 7: 862–872. - PubMed
-
- Foss EJ, Foss EJ, Radulovic D, Radulovic D, Shaffer SA, et al. (2007) Genetic basis of proteome variation in yeast. Nature Genetics 39: 1369–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

