Effect of Ca2EDTA on zinc mediated inflammation and neuronal apoptosis in hippocampus of an in vivo mouse model of hypobaric hypoxia
- PMID: 25340757
- PMCID: PMC4207758
- DOI: 10.1371/journal.pone.0110253
Effect of Ca2EDTA on zinc mediated inflammation and neuronal apoptosis in hippocampus of an in vivo mouse model of hypobaric hypoxia
Abstract
Background: Calcium overload has been implicated as a critical event in glutamate excitotoxicity associated neurodegeneration. Recently, zinc accumulation and its neurotoxic role similar to calcium has been proposed. Earlier, we reported that free chelatable zinc released during hypobaric hypoxia mediates neuronal damage and memory impairment. The molecular mechanism behind hypobaric hypoxia mediated neuronal damage is obscure. The role of free zinc in such neuropathological condition has not been elucidated. In the present study, we investigated the underlying role of free chelatable zinc in hypobaric hypoxia-induced neuronal inflammation and apoptosis resulting in hippocampal damage.
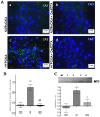
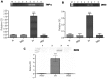
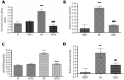
Methods: Adult male Balb/c mice were exposed to hypobaric hypoxia and treated with saline or Ca2EDTA (1.25 mM/kg i.p) daily for four days. The effects of Ca2EDTA on apoptosis (caspases activity and DNA fragmentation), pro-inflammatory markers (iNOS, TNF-α and COX-2), NADPH oxidase activity, poly(ADP ribose) polymerase (PARP) activity and expressions of Bax, Bcl-2, HIF-1α, metallothionein-3, ZnT-1 and ZIP-6 were examined in the hippocampal region of brain.
Results: Hypobaric hypoxia resulted in increased expression of metallothionein-3 and zinc transporters (ZnT-1 and ZIP-6). Hypobaric hypoxia elicited an oxidative stress and inflammatory response characterized by elevated NADPH oxidase activity and up-regulation of iNOS, COX-2 and TNF-α. Furthermore, hypobaric hypoxia induced HIF-1α protein expression, PARP activation and apoptosis in the hippocampus. Administration of Ca2EDTA significantly attenuated the hypobaric hypoxia induced oxidative stress, inflammation and apoptosis in the hippocampus.
Conclusion: We propose that hypobaric hypoxia/reperfusion instigates free chelatable zinc imbalance in brain associated with neuroinflammation and neuronal apoptosis. Therefore, zinc chelating strategies which block zinc mediated neuronal damage linked with cerebral hypoxia and other neurodegenerative conditions can be designed in future.
Conflict of interest statement
Figures






Similar articles
-
Free chelatable zinc modulates the cholinergic function during hypobaric hypoxia-induced neuronal damage: an in vivo study.Neuroscience. 2012 Jan 27;202:434-45. doi: 10.1016/j.neuroscience.2011.11.022. Epub 2011 Nov 28. Neuroscience. 2012. PMID: 22138153
-
Chronic intermittent hypoxia-induced neuronal apoptosis in the hippocampus is attenuated by telmisartan through suppression of iNOS/NO and inhibition of lipid peroxidation and inflammatory responses.Brain Res. 2015 Jan 30;1596:48-57. doi: 10.1016/j.brainres.2014.11.035. Epub 2014 Nov 24. Brain Res. 2015. PMID: 25463026
-
Nitric oxide modulates hypoxia-inducible factor-1 and poly(ADP-ribose) polymerase-1 cross talk in response to hypobaric hypoxia.J Appl Physiol (1985). 2012 Mar;112(5):816-23. doi: 10.1152/japplphysiol.00898.2011. Epub 2011 Dec 15. J Appl Physiol (1985). 2012. PMID: 22174393
-
Reactive Oxygen Species and Pulmonary Vasculature During Hypobaric Hypoxia.Front Physiol. 2018 Jul 9;9:865. doi: 10.3389/fphys.2018.00865. eCollection 2018. Front Physiol. 2018. PMID: 30050455 Free PMC article. Review.
-
Impact of Zinc on Oxidative Signaling Pathways in the Development of Pulmonary Vasoconstriction Induced by Hypobaric Hypoxia.Int J Mol Sci. 2022 Jun 23;23(13):6974. doi: 10.3390/ijms23136974. Int J Mol Sci. 2022. PMID: 35805984 Free PMC article. Review.
Cited by
-
Reg-2, A Downstream Signaling Protein in the Ciliary Neurotrophic Factor Survival Pathway, Alleviates Experimental Autoimmune Encephalomyelitis.Front Neuroanat. 2016 May 9;10:50. doi: 10.3389/fnana.2016.00050. eCollection 2016. Front Neuroanat. 2016. PMID: 27242448 Free PMC article.
-
How does zebrafish support new strategies for neuroprotection and neuroregeneration in hypoxia-related diseases?Neural Regen Res. 2016 Jul;11(7):1069-70. doi: 10.4103/1673-5374.187030. Neural Regen Res. 2016. PMID: 27630683 Free PMC article. No abstract available.
-
Disparate roles of zinc in chemical hypoxia-induced neuronal death.Front Cell Neurosci. 2015 Jan 23;9:1. doi: 10.3389/fncel.2015.00001. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25667569 Free PMC article.
-
The role of sex and ovarian hormones in hippocampal damage and cognitive deficits induced by chronic exposure to hypobaric hypoxia.Front Neurosci. 2022 Aug 8;16:953417. doi: 10.3389/fnins.2022.953417. eCollection 2022. Front Neurosci. 2022. PMID: 36003965 Free PMC article.
-
12/15-Lipoxygenase debilitates mitochondrial health in intermittent hypobaric hypoxia induced neuronal damage: An in vivo study.Redox Biol. 2022 Feb;49:102228. doi: 10.1016/j.redox.2021.102228. Epub 2021 Dec 30. Redox Biol. 2022. PMID: 34979449 Free PMC article.
References
-
- Maiti P, Singh SB, Mallick B, Muthuraju S, Ilavazhagan G (2008) High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J Chem Neuroanat 36: 227–238. - PubMed
-
- Hota SK, Barhwal K, Ray K, Singh SB, Ilavazhagan G (2008) Ceftriaxone rescues hippocampal neurons from excitotoxicity and enhances memory retrieval in chronic hypobaric hypoxia. Neurobiol Learn Mem 89: 522–532. - PubMed
-
- Udayabanu M, Kumaran D, Nair RU, Srinivas P, Bhagat N, et al. (2008) Nitric oxide associated with iNOS expression inhibits acetylcholinesterase activity and induces memory impairment during acute hypobaric hypoxia. Brain Res 1230: 138–149. - PubMed
-
- Chao WH, Askew EW, Roberts DE, Wood SM, Perkins JB (1999) Oxidative stress in humans during work at moderate altitude. J Nutr 129: 2009–2012. - PubMed
-
- Moller P, Loft S, Lundby C, Olsen NV (2001) Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J 15: 1181–1186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

