IgG1 as a potential biomarker of post-chemotherapeutic relapse in visceral leishmaniasis, and adaptation to a rapid diagnostic test
- PMID: 25340782
- PMCID: PMC4207679
- DOI: 10.1371/journal.pntd.0003273
IgG1 as a potential biomarker of post-chemotherapeutic relapse in visceral leishmaniasis, and adaptation to a rapid diagnostic test
Abstract
Background: Visceral leishmaniasis (VL), caused by protozoa of the Leishmania donovani complex, is a widespread parasitic disease of great public health importance; without effective chemotherapy symptomatic VL is usually fatal. Distinction of asymptomatic carriage from progressive disease and the prediction of relapse following treatment are hampered by the lack of prognostic biomarkers for use at point of care.
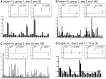
Methodology/principal findings: All IgG subclass and IgG isotype antibody levels were determined using unpaired serum samples from Indian and Sudanese patients with differing clinical status of VL, which included pre-treatment active VL, post-treatment cured, post-treatment relapsed, and post kala-azar dermal leishmaniasis (PKDL), as well as seropositive (DAT and/or rK39) endemic healthy controls (EHCs) and seronegative EHCs. L. donovani antigen-specific IgG1 levels were significantly elevated in relapsed versus cured VL patients (p<0.0001). Using paired Indian VL sera, consistent with the known IgG1 half-life, IgG1 levels had not decreased significantly at day 30 after the start of treatment (p = 0.8304), but were dramatically decreased by 6 months compared to day 0 (p = 0.0032) or day 15 (p<0.0001) after start of treatment. Similarly, Sudanese sera taken soon after treatment did not show a significant change in the IgG1 levels (p = 0.3939). Two prototype lateral flow immunochromatographic rapid diagnostic tests (RDTs) were developed to detect IgG1 levels following VL treatment: more than 80% of the relapsed VL patients were IgG1 positive; at least 80% of the cured VL patients were IgG1 negative (p<0.0001).
Conclusions/significance: Six months after treatment of active VL, elevated levels of specific IgG1 were associated with treatment failure and relapse, whereas no IgG1 or low levels were detected in cured VL patients. A lateral flow RDT was successfully developed to detect anti-Leishmania IgG1 as a potential biomarker of post-chemotherapeutic relapse.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: The design of the lateral flow rapid diagnostic tests was performed as a research collaboration with Pascal Mertens and Caroline Thunissen of the company Coris BioConcept, Belgium, without payment; none of the authors have any financial, non-financial, professional or personal conflicting interests. This collaboration does not alter our adherence to all PLOS NTDs policies on shared data and materials.
Figures


References
-
- World Health Organization (2010) Control of the Leishmaniases. World Health Organization.
-
- Sundar S, Reed SG, Singh VP, Kumar PCK, Murray HW (1998) Rapid accurate field diagnosis of Indian visceral leishmaniasis. The Lancet 351: 563–565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

