A novel function of human Pumilio proteins in cytoplasmic sensing of viral infection
- PMID: 25340845
- PMCID: PMC4207803
- DOI: 10.1371/journal.ppat.1004417
A novel function of human Pumilio proteins in cytoplasmic sensing of viral infection
Abstract
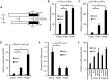
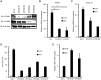
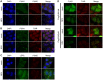
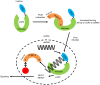
RIG-I-like receptor (RLR) plays a pivotal role in the detection of invading pathogens to initiate type I interferon (IFN) gene transcription. Since aberrant IFN production is harmful, RLR signaling is strictly regulated. However, the regulatory mechanisms are not fully understood. By expression cloning, we identified Pumilio proteins, PUM1 and PUM2, as candidate positive regulators of RIG-I signaling. Overexpression of Pumilio proteins and their knockdown augmented and diminished IFN-β promoter activity induced by Newcastle disease virus (NDV), respectively. Both proteins showed a specific association with LGP2, but not with RIG-I or MDA5. Furthermore, all of these components were recruited to NDV-induced antiviral stress granules. Interestingly, biochemical analyses revealed that Pumilio increased double-stranded (ds) RNA binding affinity of LGP2; however, Pumilio was absent in the dsRNA-LGP2 complex, suggesting that Pumilio facilitates viral RNA recognition by LGP2 through its chaperon-like function. Collectively, our results demonstrate an unknown function of Pumilio in viral recognition by LGP2.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

