Mechanisms of ventricular arrhythmias: from molecular fluctuations to electrical turbulence
- PMID: 25340965
- PMCID: PMC4342983
- DOI: 10.1146/annurev-physiol-021014-071622
Mechanisms of ventricular arrhythmias: from molecular fluctuations to electrical turbulence
Abstract
Ventricular arrhythmias have complex causes and mechanisms. Despite extensive investigation involving many clinical, experimental, and computational studies, effective biological therapeutics are still very limited. In this article, we review our current understanding of the mechanisms of ventricular arrhythmias by summarizing the state of knowledge spanning from the molecular scale to electrical wave behavior at the tissue and organ scales and how the complex nonlinear interactions integrate into the dynamics of arrhythmias in the heart. We discuss the challenges that we face in synthesizing these dynamics to develop safe and effective novel therapeutic approaches.
Keywords: chaos; multiscale dynamics; nonlinear dynamics; sudden cardiac death; ventricular arrhythmias.
Figures

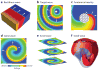
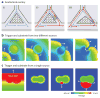
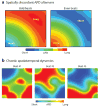
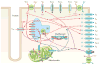
References
-
- Janse MJ, Rosen MR. History of arrhythmias. Handb Exp Pharmacol. 2006;2006:1–39. - PubMed
-
- Jalife J. Ventricular fibrillation: mechanisms of initiation and maintenance. Annu Rev Physiol. 2000;62:25–50. - PubMed
-
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–51. - PubMed
-
- Priori SG, Napolitano C. Genetics of cardiac arrhythmias and sudden cardiac death. Ann N Y Acad Sci. 2004;1015:96–110. - PubMed
-
- Marsman RF, Tan HL, Bezzina CR. Genetics of sudden cardiac death caused by ventricular arrhythmias. Nat Rev Cardiol. 2014;11:96–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

