RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer
- PMID: 25341034
- PMCID: PMC4237264
- DOI: 10.1038/cddis.2014.445
RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer
Abstract
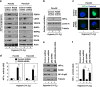
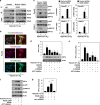
A hypoxic tumor microenvironment is characteristic of many cancer types, including one of the most lethal, pancreatic cancer. We recently demonstrated that the receptor for advanced glycation end products (RAGE) has an important role in promoting the development of pancreatic cancer and attenuating the response to chemotherapy. We now demonstrate that binding of RAGE to oncogenic KRAS facilitates hypoxia-inducible factor 1 (HIF1)α activation and promotes pancreatic tumor growth under hypoxic conditions. Hypoxia induces NF-κB-dependent and HIF1α-independent RAGE expression in pancreatic tumor cells. Moreover, the interaction between RAGE and mutant KRAS increases under hypoxia, which in turn sustains KRAS signaling pathways (RAF-MEK-ERK and PI3K-AKT), facilitating stabilization and transcriptional activity of HIF1α. Knock down of RAGE in vitro inhibits KRAS signaling, promotes HIF1α degradation, and increases hypoxia-induced pancreatic tumor cell death. RAGE-deficient mice have impaired oncogenic KRAS-driven pancreatic tumor growth with significant downregulation of the HIF1α signaling pathway. Our results provide a novel mechanistic link between NF-κB, KRAS, and HIF1α, three potent molecular pathways in the cellular response to hypoxia during pancreatic tumor development and suggest alternatives for preventive and therapeutic strategies.
Figures




Similar articles
-
RAGE maintains high levels of NFκB and oncogenic Kras activity in pancreatic cancer.Biochem Biophys Res Commun. 2017 Nov 4;493(1):592-597. doi: 10.1016/j.bbrc.2017.08.147. Epub 2017 Sep 1. Biochem Biophys Res Commun. 2017. PMID: 28867179
-
The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia.Proc Natl Acad Sci U S A. 2012 May 1;109(18):7031-6. doi: 10.1073/pnas.1113865109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509024 Free PMC article.
-
Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B.Carcinogenesis. 2011 Aug;32(8):1167-75. doi: 10.1093/carcin/bgr101. Epub 2011 Jun 3. Carcinogenesis. 2011. PMID: 21642357
-
Epidemiological-molecular evidence of metabolic reprogramming on proliferation, autophagy and cell signaling in pancreas cancer.Cancer Lett. 2015 Jan 28;356(2 Pt A):281-8. doi: 10.1016/j.canlet.2014.03.028. Epub 2014 Apr 2. Cancer Lett. 2015. PMID: 24704294 Review.
-
KRAS-related proteins in pancreatic cancer.Pharmacol Ther. 2016 Dec;168:29-42. doi: 10.1016/j.pharmthera.2016.09.003. Epub 2016 Sep 3. Pharmacol Ther. 2016. PMID: 27595930 Review.
Cited by
-
Investigating underlying molecular mechanisms, signaling pathways, emerging therapeutic approaches in pancreatic cancer.Front Oncol. 2024 Jul 17;14:1427802. doi: 10.3389/fonc.2024.1427802. eCollection 2024. Front Oncol. 2024. PMID: 39087024 Free PMC article. Review.
-
Targeting reactive oxygen species in development and progression of pancreatic cancer.Expert Rev Anticancer Ther. 2017 Jan;17(1):19-31. doi: 10.1080/14737140.2017.1261017. Epub 2016 Nov 23. Expert Rev Anticancer Ther. 2017. PMID: 27841037 Free PMC article. Review.
-
Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer.Cell Res. 2017 Jul;27(7):916-932. doi: 10.1038/cr.2017.51. Epub 2017 Apr 4. Cell Res. 2017. PMID: 28374746 Free PMC article.
-
AGE/RAGE axis regulates reversible transition to quiescent states of ALK-rearranged NSCLC and pancreatic cancer cells in monolayer cultures.Sci Rep. 2022 Jun 14;12(1):9886. doi: 10.1038/s41598-022-14272-0. Sci Rep. 2022. PMID: 35701529 Free PMC article.
-
S100s and HMGB1 Crosstalk in Pancreatic Cancer Tumors.Biomolecules. 2023 Jul 28;13(8):1175. doi: 10.3390/biom13081175. Biomolecules. 2023. PMID: 37627239 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

