Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: an open-label study
- PMID: 25341477
- PMCID: PMC4269643
- DOI: 10.1007/s13300-014-0086-7
Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: an open-label study
Abstract
Introduction: Dapagliflozin is a selective sodium glucose co-transporter 2 inhibitor that improves glycemic control and reduces body weight and systolic blood pressure in patients with type 2 diabetes mellitus (T2DM). Dapagliflozin is effective and well tolerated over 12-24 weeks in Japanese patients with T2DM. In this study, the safety and efficacy of dapagliflozin administered as monotherapy and combination therapy were assessed over 52 weeks in Japanese patients with T2DM.
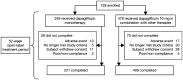
Methods: This was a 52-week open-label Phase 3 study consisting of a single treatment arm with no comparator. Dapagliflozin was administered as monotherapy (n = 249) or combination therapy (n = 479) with existing antihyperglycemic agents (sulfonylurea, glinides, metformin, alpha-glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, or glucagon-like peptide-1 receptor agonists) to Japanese patients with T2DM and inadequate glycemic control for 52 weeks. Treatment with dapagliflozin was initiated at 5 mg/day and titrated to 10 mg/day as required.
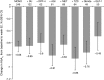
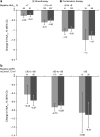
Results: Dapagliflozin administered as monotherapy or combination therapy was well tolerated. The frequency of adverse events (AEs) over 52 weeks was similar between monotherapy (79.1%) and combination therapy (72.4%) groups, and AEs were mostly mild or moderate. The incidence of hypoglycemia at 52 weeks was 2.4% in the monotherapy group and 4.0% in the combination therapy group. In patients receiving dapagliflozin as monotherapy or combination therapy, reductions from baseline to week 52 were observed in glycosylated hemoglobin (HbA1c) (-0.7% in both groups), weight (-2.6 and -2.1 kg, respectively), and systolic blood pressure (-5.2 mmHg and -3.9 mmHg). In patients with insufficient response to 5 mg/day, dapagliflozin was increased to 10 mg/day, and a further decrease in HbA1c from the pre-titration value was observed in both groups.
Conclusion: Dapagliflozin was well tolerated and effective as monotherapy or combination therapy in Japanese patients with T2DM over 52 weeks.
Figures


References
-
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124:136–145. doi: 10.7326/0003-4819-124-1_Part_2-199601011-00011. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

