Loss of β-catenin promotes chondrogenic differentiation of aortic valve interstitial cells
- PMID: 25341799
- PMCID: PMC4239156
- DOI: 10.1161/ATVBAHA.114.304579
Loss of β-catenin promotes chondrogenic differentiation of aortic valve interstitial cells
Abstract
Objective: The Wnt/β-catenin signaling pathway has been implicated in human heart valve disease and is required for early heart valve formation in mouse and zebrafish. However, the specific functions of Wnt/β-catenin signaling activity in heart valve maturation and maintenance in adults have not been determined previously.
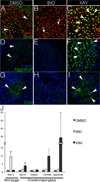
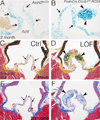
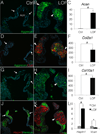
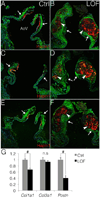
Approach and results: Here, we show that Wnt/β-catenin signaling inhibits Sox9 nuclear localization and proteoglycan expression in cultured chicken embryo aortic valves. Loss of β-catenin in vivo in mice, using Periostin(Postn)Cre-mediated tissue-restricted loss of β-catenin (Ctnnb1) in valvular interstitial cells, leads to the formation of aberrant chondrogenic nodules and induction of chondrogenic gene expression in adult aortic valves. These nodular cells strongly express nuclear Sox9 and Sox9 downstream chondrogenic extracellular matrix genes, including Aggrecan, Col2a1, and Col10a1. Excessive chondrogenic proteoglycan accumulation and disruption of stratified extracellular matrix maintenance in the aortic valve leaflets are characteristics of myxomatous valve disease. Both in vitro and in vivo data demonstrate that the loss of Wnt/β-catenin signaling leads to increased nuclear expression of Sox9 concomitant with induced expression of chondrogenic extracellular matrix proteins.
Conclusions: β-Catenin limits Sox9 nuclear localization and inhibits chondrogenic differentiation during valve development and in adult aortic valve homeostasis.
Keywords: Wnt signaling pathway; aortic valve; chondrogenesis; heart valve disease; proteoglycans.
© 2014 American Heart Association, Inc.
Figures

References
-
- Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. The Lancet. 2005;365:507–518. - PubMed
-
- Guy TS, Hill AC. Mitral valve prolapse. Annu Rev Med. 2012;63:277–292. - PubMed
-
- Hulin A, Deroanne C, Lambert C, Defraigne J-O, Nusgens B, Radermecker M, Colige A. Emerging pathogenic mechanisms in human myxomatous mitral valve: Lessons from past and novel data. Cardiovasc Pathol. 2013;22:245–250. - PubMed
-
- Lincoln J, Lange AW, Yutzey KE. Hearts and bones: Shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006;294:292–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

