Immunotoxins constructed with ribosome-inactivating proteins and their enhancers: a lethal cocktail with tumor specific efficacy
- PMID: 25341935
- PMCID: PMC4296666
- DOI: 10.2174/1381612820666140826153913
Immunotoxins constructed with ribosome-inactivating proteins and their enhancers: a lethal cocktail with tumor specific efficacy
Abstract
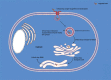
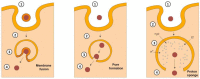
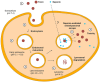
The term ribosome-inactivating protein (RIP) is used to denominate proteins mostly of plant origin, which have N-glycosidase enzymatic activity leading to a complete destruction of the ribosomal function. The discovery of the RIPs was almost a century ago, but their usage has seen transition only in the last four decades. With the advent of antibody therapy, the RIPs have been a subject of extensive research especially in targeted tumor therapies, which is the primary focus of this review. In the present work we enumerate 250 RIPs, which have been identified so far. An attempt has been made to identify all the RIPs that have been used for the construction of immunotoxins, which are conjugates or fusion proteins of an antibody or ligand with a toxin. The data from 1960 onwards is reviewed in this paper and an extensive list of more than 450 immunotoxins is reported. The clinical reach of tumor-targeted toxins has been identified and detailed in the work as well. While there is a lot of potential that RIPs embrace for targeted tumor therapies, the success in preclinical and clinical evaluations has been limited mainly because of their inability to escape the endo/lysosomal degradation. Various strategies that can increase the efficacy and lower the required dose for targeted toxins have been compiled in this article. It is plausible that with the advancements in platform technologies or improved endosomal escape the usage of tumor targeted RIPs would see the daylight of clinical success.
Figures

References
-
- Peumans WJ, Hao Q, Van Damme EJ. Ribosome-inactivating proteins from plants more than RNA N-glycosidasesκ. FASEB J. 2001;15:1493–506. - PubMed
-
- Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. - PubMed
-
- Hong X, Wang-Yi L. Cinnamonin- a versatile type II ribosome inactivating protein. Acta Biochimica et Biophysica Sinica. 2004;36:169–76. - PubMed
-
- Weng A, Thakur M, von Mallinckrodt B , et al. Saponins modulate the intracellular trafficking of protein toxins. J Control Release. 2012;164:74–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

