Acylcarnitines: potential implications for skeletal muscle insulin resistance
- PMID: 25342132
- PMCID: PMC4285541
- DOI: 10.1096/fj.14-255901
Acylcarnitines: potential implications for skeletal muscle insulin resistance
Abstract
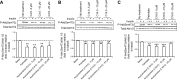
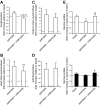
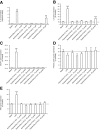
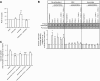
Insulin resistance may be linked to incomplete fatty acid β-oxidation and the subsequent increase in acylcarnitine species in different tissues including skeletal muscle. It is not known if acylcarnitines participate in muscle insulin resistance or simply reflect dysregulated metabolism. The aims of this study were to determine whether acylcarnitines can elicit muscle insulin resistance and to better understand the link between incomplete muscle fatty acid β-oxidation, oxidative stress, inflammation, and insulin-resistance development. Differentiated C2C12, primary mouse, and human myotubes were treated with acylcarnitines (C4:0, C14:0, C16:0) or with palmitate with or without carnitine acyltransferase inhibition by mildronate. Treatment with C4:0, C14:0, and C16:0 acylcarnitines resulted in 20-30% decrease in insulin response at the level of Akt phosphorylation and/or glucose uptake. Mildronate reversed palmitate-induced insulin resistance concomitant with an ∼25% decrease in short-chain acylcarnitine and acetylcarnitine secretion. Although proinflammatory cytokines were not affected under these conditions, oxidative stress was increased by 2-3 times by short- or long-chain acylcarnitines. Acylcarnitine-induced oxidative stress and insulin resistance were reversed by treatment with antioxidants. Results are consistent with the conclusion that incomplete muscle fatty acid β-oxidation causes acylcarnitine accumulation and associated oxidative stress, raising the possibility that these metabolites play a role in muscle insulin resistance.
Keywords: fatty acid β-oxidation; inflammation; mitochondria; myotubes; oxidative stress.
© FASEB.
Figures

References
-
- Kelley D. E., He J., Menshikova E. V., and Ritov V. B. (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950 - PubMed
-
- Mogensen M., Sahlin K., Fernström M., Glintborg D., Vind B. F., Beck-Nielsen H., and Højlund K. (2007) Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56, 1592–1599 - PubMed
-
- Muoio D. M., Noland R. C., Kovalik J. P., Seiler S. E., Davies M. N., DeBalsi K. L., Ilkayeva O. R., Stevens R. D., Kheterpal I., Zhang J., Covington, J. D., Bajpeyi, S., Ravussin, E., Kraus, W., Koves, T. R., and Mynatt, R. L. (2012) Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 15, 764–777 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

