Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel?
- PMID: 25342630
- PMCID: PMC4657553
- DOI: 10.1038/nrc3803
Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel?
Abstract
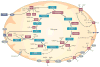
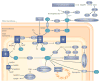
Mitochondria cooperate with their host cells by contributing to bioenergetics, metabolism, biosynthesis, and cell death or survival functions. Reactive oxygen species (ROS) generated by mitochondria participate in stress signalling in normal cells but also contribute to the initiation of nuclear or mitochondrial DNA mutations that promote neoplastic transformation. In cancer cells, mitochondrial ROS amplify the tumorigenic phenotype and accelerate the accumulation of additional mutations that lead to metastatic behaviour. As mitochondria carry out important functions in normal cells, disabling their function is not a feasible therapy for cancer. However, ROS signalling contributes to proliferation and survival in many cancers, so the targeted disruption of mitochondria-to-cell redox communication represents a promising avenue for future therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Embley TM, Martin W. Eukaryotic evolution, changes and challenges. Nature. 2006;440:623–630. - PubMed
-
- Martin W, Hoffmeister M, Rotte C, Henze K. An overview of endosymbiotic models for the origins of eukaryotes, their ATP-producing organelles (mitochondria and hydrogenosomes), and their heterotrophic lifestyle. Biol Chem. 2001;382:1521–1539. - PubMed
-
- Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nature Rev Genet. 2004;5:123–135. - PubMed
-
- Adams KL, Palmer JD. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol. 2003;29:380–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

