Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution
- PMID: 25342811
- PMCID: PMC4336192
- DOI: 10.1126/science.1257998
Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution
Abstract
Although fluorescence microscopy provides a crucial window into the physiology of living specimens, many biological processes are too fragile, are too small, or occur too rapidly to see clearly with existing tools. We crafted ultrathin light sheets from two-dimensional optical lattices that allowed us to image three-dimensional (3D) dynamics for hundreds of volumes, often at subsecond intervals, at the diffraction limit and beyond. We applied this to systems spanning four orders of magnitude in space and time, including the diffusion of single transcription factor molecules in stem cell spheroids, the dynamic instability of mitotic microtubules, the immunological synapse, neutrophil motility in a 3D matrix, and embryogenesis in Caenorhabditis elegans and Drosophila melanogaster. The results provide a visceral reminder of the beauty and the complexity of living systems.
Copyright © 2014, American Association for the Advancement of Science.
Figures
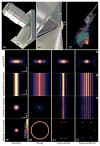
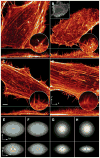




Comment in
-
Microscopy: Advancing imaging.Nat Rev Mol Cell Biol. 2014 Dec;15(12):769. doi: 10.1038/nrm3910. Nat Rev Mol Cell Biol. 2014. PMID: 25415507 No abstract available.
-
Light microscopy with lattices.Nat Methods. 2014 Dec;11(12):1191. doi: 10.1038/nmeth.3196. Nat Methods. 2014. PMID: 25584377 No abstract available.
References
-
- Murphy DB, Davidson MW. Fundamentals of light microscopy and electronic imaging. 2. Wiley-Blackwell; Hoboken, N.J: 2013. p. xiii.p. 538.
-
- Stephens DJ, Allan VJ. Light microscopy techniques for live cell imaging. Science. 2003;300:82–86. - PubMed
-
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305:1007–1009. - PubMed
-
- Krzic U, Gunther S, Saunders TE, Streichan SJ, Hufnagel L. Multiview light-sheet microscope for rapid in toto imaging. Nature methods. 2012;9:730–733. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

