Efficacy and safety of combining olodaterol Respimat(®) and tiotropium HandiHaler(®) in patients with COPD: results of two randomized, double-blind, active-controlled studies
- PMID: 25342898
- PMCID: PMC4206204
- DOI: 10.2147/COPD.S72482
Efficacy and safety of combining olodaterol Respimat(®) and tiotropium HandiHaler(®) in patients with COPD: results of two randomized, double-blind, active-controlled studies
Abstract
Background: Combining bronchodilators with different mechanisms of action may improve efficacy and reduce risk of side effects compared to increasing the dose of a single agent in chronic obstructive pulmonary disease (COPD). We investigated this by combining two long-acting bronchodilators: once-daily muscarinic antagonist tiotropium and once-daily β2-agonist olodaterol.
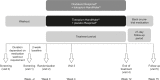
Methods: Two replicate, double-blind, randomized, 12-week studies (ANHELTO 1 [NCT01694771] and ANHELTO 2 [NCT01696058]) evaluated the efficacy and safety of olodaterol 5 μg once daily (via Respimat(®)) combined with tiotropium 18 μg once daily (via HandiHaler(®)) versus tiotropium 18 μg once daily (via HandiHaler(®)) combined with placebo (via Respimat(®)) in patients with moderate to severe COPD. Primary efficacy end points were area under the curve from 0-3 hours of forced expiratory volume in 1 second (FEV1 AUC0-3) and trough FEV1 after 12 weeks (for the individual trials). A key secondary end point was health status by St George's Respiratory Questionnaire (SGRQ) total score (combined data set).
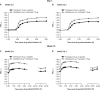
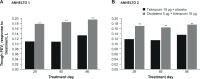
Results: Olodaterol + tiotropium resulted in significant improvements over tiotropium + placebo in FEV1 AUC0-3 (treatment differences: 0.117 L [P<0.001], ANHELTO 1; 0.106 L [P<0.001], ANHELTO 2) and trough FEV1 (treatment differences: 0.062 L [P<0.001], ANHELTO 1; 0.040 L [P=0.0029], ANHELTO 2); these were supported by secondary end points. These effects translated to improvements in SGRQ total scores (treatment difference -1.85; P<0.0001). The tolerability profile of olodaterol + tiotropium was similar to tiotropium monotherapy.
Conclusion: These studies demonstrated that olodaterol (Respimat(®)) and tiotropium (HandiHaler(®)) provided bronchodilatory effects above tiotropium alone in patients with COPD. In general, both treatments were well tolerated.
Keywords: bronchodilator; long-acting beta2-agonist; long-acting muscarinic antagonist; olodaterol Respimat®; tiotropium HandiHaler®.
Figures


References
-
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. - PubMed
-
- National Heart, Lung, and Blood Institute [homepage on the Internet] NHLBI Factbook. 4. Disease Statistics [updated 2006] [Accessed March 21, 2014]. Available from: http://www.nhlbi.nih.gov/about/factbook-06/chapter4data.htm.
-
- Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet] Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. [Accessed March 5, 2014]. Updated 2013 [updated 2013]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf.
-
- Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217–224. - PubMed
-
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

